Name: Spermidine
Class: biogenic polyamine
Alias(es): CAS#: 124-20-9
Background: Spermidine is a naturally occurring polyamine that was first isolated from semen, but actually exists in all eukaryotic organisms’ cells and declines in quantity with age.[1] Spermidine levels can be increased by a diet rich in spermidine rich foods, taking spermidine supplements, or taking pre or probiotics such as Bifidobacterium LKM512[2]. Intriguingly, increased spermidine intake is associated with a variety of improved physiological biomarkers and increased health-span and lifespan.
Evidence that spermidine works in human clinical studies for aging?:
NCT03094546: (Completed May 2020). Title: Polyamine-enriched Diet in Elderly Individuals With Subjective Cognitive Decline (SmartAge)
Summary: The study will investigate whether a polyamine-enriched dietary supplementation (through capsule intake) could provide positive effects on cognitive function and biomarkers of elderly individuals (60-90 years old) with subjective cognitive decline (SCD). (They were given a daily dose of 0.9 mg for 12 months.)
Result: Longer-term spermidine supplementation with an increased daily supply of spermidine by about 10% did not modify memory and other biomarkers in a group of older adults at risk for Alzheimer disease. See full details here.
Historical Prospective Clinical Trial: NCT03378843 on ClinicalTrials.gov:
Summary: This prospective Bruneck Study clinical trial used dietician-administered questionnaires to determine the association between spermidine dietary intake (not supplement intake) and all-cause mortality. It was carried out from 1995-2010 in a community-based cohort of 829 individuals ages of 45 to 84 years, half of which were females, and included mortality follow-up through 2015.[3]
Results: Higher spermidine intake was significantly associated with reduced mortality: The age-, sex- and caloric ratio–adjusted HR for all-cause death per 1-SD higher spermidine intake was 0.74 (95% CI: 0.66, 0.83; P < 0.001). Further adjustment for lifestyle factors, established predictors of mortality, and other dietary features yielded an HR of 0.76 (95% CI: 0.67, 0.86; P < 0.001).
This was independently validated in the Salzburg Atherosclerosis Prevention Program in Subjects at High Individual Risk (SAPHIR) Study. Overall, the difference in mortality risk between the top and bottom third of spermidine intakes was similar to that associated with a 5.7-y (95% CI: 3.6, 8.1 y) younger age.
Ongoing trials: None for aging specifically. One for people with hypertension: NCT04405388
Evidence Spermidine works in preclinical studies for aging:
Spermidine administration has been shown to induce autophagy and extend lifespan and healthspan in multiple model organisms both in vitro and in vivo, i.e. yeast, c. elegans (a microscopic worm species), drosophila m. (species of fruit fly)[4,5], and mice[6], as well as in human immune cells (PBMC)[4].
Additionally, a study found that six months administration of spermidine in drinking water of aged mice resulted in benefits in multiple age-associated phenotypes. It protected against hair-loss, improved brain-glucose metabolism (similar to that in younger mice), decreased the severity and incidence of age-related inflammation and/or pathologies in the heart, kidney, and liver, and significantly prevented shortening of telomeres in heart tissue.[7] In each of the above studies, spermidine supplementation did not result in reduced food intake or weight loss.
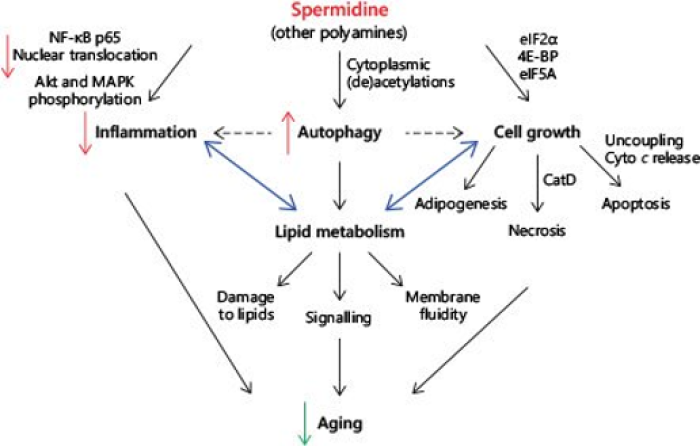
Figure from https://www.karger.com/Article/Fulltext/356748
The main proposed mechanism by which spermidine exerts these beneficial effects on lifespan is by causing autophagy[4,8], a process by which damaged and junk material is cleared from cells, followed by the debris being removed from the body. In fact, removing autophagy from the equation via deletion of a required gene, eliminated the life-span extending effect of spermidine in cells of yeast, worms, and flies[4] and failed to provide cardio-protection in mice[6]. However, there are several other pathways that spermidine acts upon, which may also be responsible for its demonstrated ability to extend lifespan and healthspan in-vivo. These include, as shown above, suppressing inflammation, altering lipid metabolism, and regulating cell growth and death[8]. Experiments have also shown that spermidine has the ability to modify reactive oxygen species[7,9], regulate DNA replication, transcription, and translation, alter mitochondrial function, improve proteostasis, and increase hypusination (the formation of hypusine, a crucial amino acid involved in translation in eukaryotes)[7].
Safety Concerns?
No adverse effects were demonstrated during life-long administration of spermidine in mice.[6]
In a phase II clinical trial to assess safety in humans, spermidine administration at an oral dosage of 1.2mg per day over a 3 month period was determined to be safe. The preclinical toxicity study carried out in mice found no morbidities, no changes in behavior, and no evidence of tumorigenic or fibrotic events, even at extreme doses (oral 50g/kg body weight)[10]. Thus, there are no known side effects of oral spermidine supplementation. A currently recruiting clinical trial will use 4mg/day.[11]
Literature Cited
1. Madeo, F., Bauer, M. A., Carmona-Gutierrez, D., & Kroemer, G. (2018). Spermidine: A physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy, 15(1), 165–168. https://doi.org/10.1080/15548627.2018.1530929
2. Madeo, F., Hofer, S. J., Pendl, T., Bauer, M. A., Eisenberg, T., Carmona-Gutierrez, D., & Kroemer, G. (2020). Nutritional Aspects of Spermidine. Annual Review of Nutrition, 40, 135–159. https://doi.org/10.1146/annurev-nutr-120419-015419
3. Kiechl, S., Pechlaner, R., Willeit, P., Notdurfter, M., Paulweber, B., Willeit, K., Werner, P., Ruckenstuhl, C., Iglseder, B., Weger, S., Mairhofer, B., Gartner, M., Kedenko, L., Chmelikova, M., Stekovic, S., Stuppner, H., Oberhollenzer, F., Kroemer, G., Mayr, M., … Willeit, J. (2018). Higher spermidine intake is linked to lower mortality: A prospective population-based study. The American Journal of Clinical Nutrition, 108(2), 371–380. https://doi.org/10.1093/ajcn/nqy102
4. Eisenberg, T., Knauer, H., Schauer, A., Büttner, S., Ruckenstuhl, C., Carmona-Gutierrez, D., Ring, J., Schroeder, S., Magnes, C., Antonacci, L., Fussi, H., Deszcz, L., Hartl, R., Schraml, E., Criollo, A., Megalou, E., Weiskopf, D., Laun, P., Heeren, G., … Madeo, F. (2009). Induction of autophagy by spermidine promotes longevity. Nature Cell Biology, 11(11), 1305–1314. https://doi.org/10.1038/ncb1975
5. Morselli, E., Mariño, G., Bennetzen, M. V., Eisenberg, T., Megalou, E., Schroeder, S., Cabrera, S., Bénit, P., Rustin, P., Criollo, A., Kepp, O., Galluzzi, L., Shen, S., Malik, S. A., Maiuri, M. C., Horio, Y., López-Otín, C., Andersen, J. S., Tavernarakis, N., … Kroemer, G. (2011). Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. The Journal of Cell Biology, 192(4), 615–629. https://doi.org/10.1083/jcb.201008167
6. Eisenberg, T., Abdellatif, M., Schroeder, S., Primessnig, U., Stekovic, S., Pendl, T., Harger, A., Schipke, J., Zimmermann, A., Schmidt, A., Tong, M., Ruckenstuhl, C., Dammbrueck, C., Gross, A. S., Herbst, V., Magnes, C., Trausinger, G., Narath, S., Meinitzer, A., … Madeo, F. (2016). Cardioprotection and lifespan extension by the natural polyamine spermidine. Nature Medicine, 22(12), 1428–1438. https://doi.org/10.1038/nm.4222
7. Wirth, A., Wolf, B., Huang, C.-K., Glage, S., Hofer, S. J., Bankstahl, M., Bär, C., Thum, T., Kahl, K. G., Sigrist, S. J., Madeo, F., Bankstahl, J. P., & Ponimaskin, E. (2021). Novel aspects of age-protection by spermidine supplementation are associated with preserved telomere length. GeroScience, 43(2), 673–690. https://doi.org/10.1007/s11357-020-00310-0
8. Minois, N. (2014). Molecular Basis of the ‘Anti-Aging’ Effect of Spermidine and Other Natural Polyamines—A Mini-Review. Gerontology, 60(4), 319–326. https://doi.org/10.1159/000356748
9. Jeong, J.-W., Cha, H.-J., Han, M. H., Hwang, S. J., Lee, D.-S., Yoo, J. S., Choi, I.-W., Kim, S., Kim, H.-S., Kim, G.-Y., Hong, S. H., Park, C., Lee, H.-J., & Choi, Y. H. (2018). Spermidine Protects against Oxidative Stress in Inflammation Models Using Macrophages and Zebrafish. Biomolecules & Therapeutics, 26(2), 146–156. https://doi.org/10.4062/biomolther.2016.272
10. Schwarz, C., Stekovic, S., Wirth, M., Benson, G., Royer, P., Sigrist, S. J., Pieber, T., Dammbrueck, C., Magnes, C., Eisenberg, T., Pendl, T., Bohlken, J., Köbe, T., Madeo, F., & Flöel, A. (2018). Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging (Albany NY), 10(1), 19–33. https://doi.org/10.18632/aging.101354