Name: Quercetin
Class: flavonol supplement
Alias(es): CAS# 117-39-5, 5,7,3′,4′-flavon-3-ol; Sophoretin; Meletin; Quercetine; Xanthaurine; Quercetol; Quercitin; Quertine; Bio-Quercetin; Flavin meletin
Background: Quercetin is a flavonoid found naturally in many fruits and vegetables, and most abundantly in onions, grapes, berries, cherries, broccoli, and citruses[1]. Supplements can be taken to achieve much higher levels of quercetin than can be obtained from dietary sources. Quercetin is effective at restoring NAD levels, and targeting senescent cells of certain cell types whereas dasatinib, a synthetic senolytic drug, is effective at clearing senescent cells of other cell types[2]. Thus, although they can be taken individually, quercetin is generally administered in combination with the prescription drug dasatinib (D+Q) in preclinical and clinical trials to achieve a broader senolytic effect. Although it is yet unknown whether D+Q can improve health and lifespan in aging humans, clinical trials will soon be underway (see section immediately below), and D+Q has shown promise in this regard in preclinical trials both in vitro in human cells and in vivo in mice and rats (see preclinical studies section). Lastly, it is important to consider that quercetin is considered Generally Recognized As Safe (GRAS) by the FDA[3] and has been safely used at high doses in short-term (3 months) human clinical trials[4]. However, there is limited data for long-term administration of quercetin in humans and toxicity studies in rats suggest that long-term administration of quercetin, especially at higher doses, may be toxic to the kidneys and carcinogenic[5].
Is there evidence it works in humans for aging?
ClinicalTrials.gov ID And Reference | Trial Status | Title | Summary | Results |
Completed (August 2012) | Can Fish Oil and Phytochemical Supplements Mimic Anti-Aging Effects of Calorie Restriction? | Participants (40-60 yrs.) took 10 supplements each day (curcumin, fish oil, resveratrol, sesamin, Acetyl-L-carnitine, lipoic acid, green and black teas, quercetin, pomegranate, cinnamon bark) for 6 mo. | Multiple dietary supplements do not affect metabolic and cardio-vascular health | |
Completed (December 2014) | Hypoxia-inducible Transcription Factor 1 (HIF-1) in Vascular Aging | Participants (18-75 yrs.) took 500 mg of Quercetin or placebo once per day for 6 mo. | Cannot find results | |
Active, not Recruiting | The Safety and Effectiveness of Quercetin and Dasatinib on the Epigenetic Aging Rates in Healthy Individuals | This prospective non-randomized clinical study will involve participants (≥ 40 yrs.old) taking 500mg Quercetin and 50mg Dasatinib oral capsules on Monday, Tuesday, Wednesday (3 days in a row) per month for the duration of 6 mo. It will evaluate the change from baseline in epigenetic age test results (to be obtained via the illumina 850k epic array). | ||
Not Yet Recruiting | Pilot Study to Test the Safety and Efficacy of Metformin, Dasatinib, Rapamycin and Nutritional Supplements (Bio-quercetin; Bio-fisetin; Glucosamine; Nicotinamide Riboside; Trans-resveratrol) in Reducing Clinical Measures of Aging in Older Adults | This study is also known as the VIAging Deceleration Trial Using Metformin, Dasatinib, Rapamycin and Nutritional Supplements. It will be accepting healthy volunteers ≥ 65 years old.
This study will involve first taking metformin and gradually increasing the dose. Then dasatinib, bio-quercetin, and bio-fisetin will be added. Then glucosamine, NR, and resveratrol will be added. And lastly rapamycin will be taken, at which point the subjects will continue on this intervention (taking all of these compounds) for 12 months. The study will evaluate: adverse effects of treatments, DEXA scans of visceral adipose tissue, systolic blood pressure, senescent cell levels, glucose control, and DNA methylation via GrimAge. Estimated study completion date is December 2023. |
Is there evidence it works in preclinical studies for aging?
Quercetin-only preclinical studies: When quercetin is used alone, it has been found to: restore NAD levels by inhibiting an NAD consuming enzyme (CD38), reduce senescent cell levels, oxidative stress, and inflammation, and improve bone repair. See table below for references and details.
Model | Vivo/Vitro | Outcome | Reference |
Recombinant human CD38, mouse embryonic fibroblast cells (MEFs) | In vitro | Quercetin inhibits human CD38 NADase activity, and also inhibits CD38 in MEFs, ameliorating the age-dependent decline in NAD levels in vitro. | |
Mice | In vitro |
| |
Rats | Vivo/Vitro |
|
Dasatinib+Quercetin (D+Q) preclinical studies: Benefits of D+Q for aging (as demonstrated in preclinical studies) include reduction of senescent cell burden and inflammatory markers, and improvements in cardiovascular health, gut microbiome, fatty liver disease, bone health and disc degeneration, strength and physical health, and cognitive health. See table below for references and details.
Model | Vivo/Vitro | Outcome | Reference |
Mice and human cells | Vivo/Vitro |
| |
Mice | Vivo | Chronic D+Q (oral) cleared some types of senescent cells, and alleviated vasomotor dysfunction in naturally aged mice and mice with established atherosclerosis via modulation of nitric oxide (NO). D+Q reduced aortic intimal plaque calcification but did not affect plaque fibrosis. | |
Mice (aged) | In Vivo | D+Q treatment significantly reduced intestinal senescence (as measured by markers p16Ink4a and p21Cip1) and inflammation (as measured by markers Cxcl1, Il1β, Il6, Mcp1, and Tnfα), and it altered the gut microbiome composition in aged mice (suggests it may treat age-related dysbiosis). Placebo-controlled. | |
Mice (INK-ATTAC) | Vivo | Found that accumulation of senescent cells promotes accumulation of fat in the liver and steatosis (liver fat build-up). Thus aging is a contributing factor in causing non-alcoholic fatty liver disease (NAFLD). D+Q effectively reduced overall liver steatosis by removing senescent cells. Mechanism: mitochondria in senescent cells inefficiently metabolize fatty acids, but senolytic therapy with D+Q therapeutically addresses this. | |
Mice, human cells | Vivo/Vitro |
| |
Mice C57BL/6 (aged) | In Vivo |
| |
Mice (aged) | Vivo/Vitro | D+Q improved bone forming capacity of bone marrow mesenchymal stem cells (BSMCs) from aged mice both in vitro and in vivo. | |
Mice (C57Bl/6J) | Vivo/Vitro | D+Q injected bi-weekly into young (3mo) and old (20mo) mice following injury resulted in blunted muscle regeneration in young mice, but improved muscle regeneration in old mice. Additionally, D+Q treated old mice had significantly less senescence levels than old controls. In vitro experiments showed that D+Q improved myogenic progenitor cell proliferation. | |
Mice (INK-ATTAC) | Vivo/Vitro | There is an age-dependent increase in p16Ink4a-positive senescent cells and SASP (inflammation) in brains of mice. Aged mice also experience cognitive decline.Study used INK-ATTAC mice, in which a drug (AP20187) can eliminate p16Ink4a-positive senescent cells. Both D+Q (given orally) and the AP20187 drug (via injection) resulted in a decrease in p16Ink4a-positive microglia and SASP factors in the hippocampus brain region of naturally aged mice, and both treatments significantly improved cognitive function. This suggests that brain inflammation and cognitive decline may be successfully treated with systemic senescent-cell clearance via oral D+Q. | |
Mice (male) | Vivo | D+Q once per month for 4 months significantly lowered senescence levels (p16ink4a mRNA) in bone and the percentage of senescent osteocytes in aged (20mo old) mice vs controls. Significantly cleared senescent cells in 24mo old treated mice, improved spine and femur bone morphology, and improved femur thickness and strength. Lower osteoclast numbers and bone resorption were observed. | |
Mice (SAMP10) | Vivo/Vitro |
| |
Wistar rats | Vivo |
|
Quercetin Mechanisms:
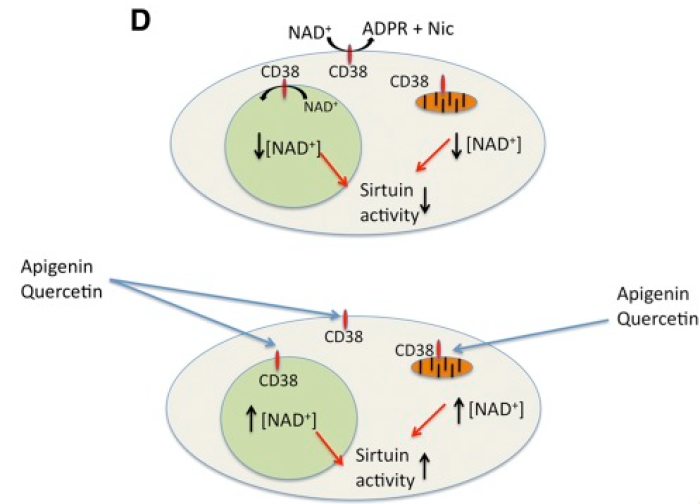
Maintaining NAD+ Homeostasis:
The figure above is from Escande et al., 2013. It shows the mechanism by which the senolytic drugs Apigenin and Quercetin improve NAD levels that have fallen due to aging. CD38 naturally causes low NAD+ levels within cells, which results in low sirtuin activity (nutrient sensing enzymes which have been linked to improved healthspan and lifespan when active). However, both Apigenin and Quercetin inhibit CD38’s NADase activity, leading to higher levels of NAD intracellularly[6].
Senolytic Properties:
The figure above is from a review by Martel et al., 2020. It shows the pathways inhibited by senolytic supplements, such as Quercetin, and drugs, such as Dasatinib, that lead to reduced viability of senescent cells and/or apoptosis of senescent cells.
Quercetin is known to selectively target senescent endothelial cells, the cells that line blood vessels, the heart, and lymph nodes, whereas the drug Dasatinib is known to target senescent pre-adipocytes[7]. Thus, as mentioned earlier, dasatinib and quercetin have complimentary senolytic effects, enabling the targeting of a broader range of senescent cells than either alone, and are generally administered together in a combination known as D+Q[7]. In fact, some senescent cell types, such as mouse fibroblast cells, do not respond to either D or Q alone, but are effectively cleared when the combination of D+Q is used[2].
Many of the benefits of D+Q for aging are due to this senolytic effect, which results in less systemic inflammation and damage by reducing levels of Senescence-Associated Secretory Phenotype (SASP). The mechanism by which D+Q removes senescent cells, while not affecting non-senescent cells, is by inhibiting certain Senescent Cell Anti-Apoptotic Pathways (SCAPs), which are upregulated by senescent cells to avoid apoptosis, programmed destruction that would otherwise be activated by the immune system in response to senescent cells’ inflammatory SASP[8]. Several of the SCAPs inhibited by Quercetin are BCL-2/BCL-XL, PI3K/AKT, and p53/p21/serpine, whereas Dasatinib targets the ephrin/src tyrosine kinase pathway and dependence receptors[9]. Different SCAPs are involved in different types of senescent cells, which explains why Quercetin and Dasatinib selectively target different cell types.
Are there known safety concerns?
Quercetin is considered Generally Recognized As Safe (GRAS) by the FDA.
There is no reported toxicity attributable to quercetin in human studies, but long-term data is limited.
In a 2018 human study involving treating idiopathic pulmonary fibrosis (IPF) patients (ages 55-84) with intermittent DQ (D:100 mg/day, Q:1250 mg/day, three-days/week over three-weeks), all patients were retained for the study course with no D+Q discontinuation. Although there was no placebo arm, the adverse events reported were consistent with previous placebo arms in trials for IPF, and there was only one serious adverse event (pneumonia and edema) which was completely resolved after hospitalization. There were no changes in laboratory tests to indicate any liver or kidney toxicity and pulmonary function did not change.
Results of Long-term (2 years) daily administration of Quercetin (at 40, 400, and 1,900 mg/kg) rat toxicity study:
In male rats, there was some evidence of carcinogenicity.
In female rats, there was no evidence of carcinogenicity at all doses tested.
In male rats, kidney dysfunction (specifically the incidence of renal tubule hyperplasia and the severity of nephropathy) were increased in a dose-dependent manner.
Literature Cited:
1. Anand David, A. V., Arulmoli, R., & Parasuraman, S. (2016). Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacognosy Reviews, 10(20), 84–89. https://doi.org/10.4103/0973-7847.194044
2. Zhu, Y., Tchkonia, T., Pirtskhalava, T., Gower, A. C., Ding, H., Giorgadze, N., Palmer, A. K., Ikeno, Y., Hubbard, G. B., Lenburg, M., O’Hara, S. P., LaRusso, N. F., Miller, J. D., Roos, C. M., Verzosa, G. C., LeBrasseur, N. K., Wren, J. D., Farr, J. N., Khosla, S., … Kirkland, J. L. (2015). The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell, 14(4), 644–658. https://doi.org/10.1111/acel.12344
8. Kirkland, J. L., & Tchkonia, T. (2020). Senolytic drugs: From discovery to translation. Journal of Internal Medicine, 288(5), 518–536. https://doi.org/10.1111/joim.13141
9. Kirkland, J. L., & Tchkonia, T. (2017). Cellular Senescence: A Translational Perspective. EBioMedicine, 21, 21–28. https://doi.org/10.1016/j.ebiom.2017.04.013