Name: NR (Nicotinamide Riboside)
Class: NAD+ precursor Supplement
Alias(es): CAS# 1341-23-7, Brand Names: Niagen, TruNiagen
Background: Nicotinamide Riboside (NR) is a derivative of vitamin B3 and is an NAD+ precursor supplement that has been demonstrated to raise NAD+ levels in humans and model organisms (see studies in sections below). This is important because NAD+ levels fall naturally with age and may be implicated in some of aging’s negative health effects[1]. What sets NR apart from Niacin (NA) is that it has a slightly different structure, enabling the cell to skip an otherwise necessary step in the production of NAD+ and instead use the “salvage pathway[2,3].” This is perhaps the reason that NR does not have some of the common side-effects associated with Niacin, i.e. flushing[2]. It is yet unknown whether the health benefits seen in preclinical studies (see section on preclinical studies), are translatable to humans. NR is sold as a supplement, and given its established safety profile from clinical trials, may be worth a try.
Is there evidence it works in humans for aging?
Completed Clinical Trials:
NCT02921659: (Completed October 2016), Title: Safety & Efficacy of Nicotinamide Riboside Supplementation for Improving Physiological Function in Middle-Aged and Older Adults
Summary of trial: This was a 2 × 6-week randomized, double-blind, placebo-controlled crossover clinical trial. Subjects ingested nicotinamide riboside chloride (NIAGEN®; 500 mg, twice per day; ChromaDex, Inc.) and placebo capsules for 6 weeks each in a randomly determined order. Study subjects were sixty healthy middle-aged and older men and women between the ages of 55 and 79 years
Results here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5876407/ Summary of results:
NR treatment was well tolerated with no adverse effects.
NR supplementation effectively increased levels of NAD+ (~60% vs placebo) and levels of related metabolites in peripheral blood mononuclear cells (PBMCs)
No improvements in measures of cardiovascular health (e.g. decreased mean systolic and diastolic blood pressure) after controlling for other factors, and no statistically significant improvement in health or fitness parameters were found. Authors state that longer term studies with larger cohorts are needed.
NCT03501433: (Completed December 1, 2019), Title: Effects of Nicotinamide Riboside on Metabolism and Vascular Function
Summary of trial: Determined the effects of 7 days of Nicotinamide Riboside (NR) (250 mg capsules 2x/day) or placebo on metabolism and vascular function following high-fat meal. Differences between young (18-35) and older (60-75) adults were determined.
Results: No significant effects of NR supplementation for 1 week were identified in terms of microvascular function, plasma triglycerides, plasma glucose, or postprandial oxidative stress. See for details: https://dr.lib.iastate.edu/server/api/core/bitstreams/8c7860c8-457a-4c42-aeeb-c363df33af90/content
A study published in Cell Reports in August 2019 involved a placebo-controlled, randomized, double-blind, crossover trial of supplementing 1000 mg of NR per day, for 21 days, in 12 healthy, marginally overweight, aged men (median 75 years old).
Results:
NR augments the aged human skeletal muscle NAD+ metabolome. Meaning that oral NR supplementation is effective at making NAD+ available to human skeletal muscle.
NR induces a transcriptional signature (downregulates genes associated with energy metabolism in skeletal muscle)
Levels of several circulating inflammatory cytokines were lowered by NR supplementation:NR significantly decreased the levels of the interleukins IL-6, IL-5, and IL-2 and tumor necrosis factor alpha (TNF-α), compared to baseline, and with the exception of TNF-α, all of these were also significant compared to placebo. The authors believe that there was a carry-over effect beyond the washout period of NR in the placebo group for TNF-α.
No effect on mitochondrial function or systemic cardiometabolic parameters.
No effect on hand grip strength
Another study, published in Scientific Reports found that NR was safe, well-tolerated at up to 1000 mg per day for 8 weeks, and increased NAD+ levels.
Results: Did not find any significant effects on any health measures in this trial. Those included vitals, blood counts and hematology, high density lipoprotein cholesterol (HDL-C), LDL-C, triglycerides, total cholesterol, HCY levels (which indicates NR supplementation does not cause a shortage of methyl groups), resting energy expenditure (REE), blood levels of branched-chain amino acids, or hsCRP. This study involved healthy men and non-pregnant, non-breastfeeding women (40–60 years of age).
Table of currently recruiting & not yet recruiting human clinical trials of NR for aging
Is there evidence it works in preclinical studies for aging?
Many studies on the in vivo effects of NR have been conducted in mice and rats:
Table 2 in the 2019 review article “NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR” by Rajman et al. from the journal Cell lists many of them. Direct link to Table 2 here.
Research has continued after the review article was published, and here are some of the more recent findings in mice/rats: rejuvenation of aged gut stem cells (intestinal stem cells or ISCs), improved ear health and hearing in mouse models of cochrane syndrome, and protected against aging-induced liver dysfunction.
Some additional findings are listed in Table 1 from a 2020 review article published in the journal Nature.
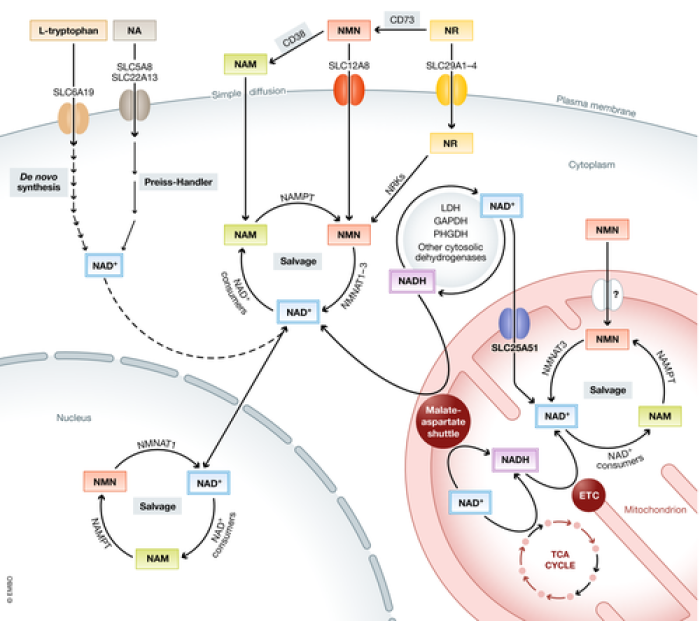
Mechanism: The mechanism by which these supplements are thought to mediate these benefits is by raising levels of NAD+, a crucial molecule in cellular metabolism and respiration, levels of which fall naturally with age. Raising NAD+ (NAD+ repletion) is also known to activate sirtuins, which stabilize telomeres and promote healthy aging by signaling nutrient scarcity and catabolism. The specific pathway, clearly distinct from niacin, by which NR is utilized by the cell to produce NAD+ can be seen in the diagram below from a 2021 review article by Zapata-Pérez et al. published in the journal EMBO Molecular Medicine:
For a depiction of the current understanding of the mechanism by which NR, via increased NAD+ levels, exerts its effects, see the Figure 4 below from a 2020 review article by Covarrubias et al., published in the journal Nature:
Are there known safety concerns?
In the human clinical trials listed in the first section of this report, no adverse effects of NR administration were found at up to 1000 mg/day for the time periods tested.
A study performed on mice for toxicity of NR supplementation found the following:
90-day toxicity study in mice: There were no adverse effects at 300 mg/kg/day. However, at 1000 mg/kg/day, there were statistically significant increases in liver and kidney weights.
Acute toxicity study: No mortality or adverse changes were seen in mice at a single dose of 5000 mg/kg.
Literature Cited: