Name: NMN (Nicotinamide Mononucleotide)
Class: NAD+ precursor Supplement
Alias(es): CAS# 1094-61-7
Background: Nicotinamide mononucleotide (NMN) is marketed as an NAD+ precursor supplement for humans because it has been shown to restore NAD+ levels by enhancing biosynthesis of NAD+ in multiple preclinical studies[1,2]. NMN is one of the more recent NAD+ supplements, and is a promising treatment for many aspects of aging, as evidenced by the myriad health benefits seen in preclinical studies in mice (see section on preclinical studies). NMN, like nicotinamide riboside (NR), is slightly different from niacin (NA) and uses distinct biological pathways to increase NAD biogenesis[3]. Likely because of this, NMN also does not have the side-effects associated with NA, i.e. flushing.
Is there evidence it works in humans for aging?
Completed Clinical Trials:
A study titled “Effect of 12-Week Intake of Nicotinamide Mononucleotide on Sleep Quality, Fatigue, and Physical Performance in Older Japanese Adults: A Randomized, Double-Blind Placebo-Controlled Study” was published February 6, 2022.
Summary: 108 participants (men and women ages 65 and older) took 250 mg/day of NMN either in the morning (AM group) or evening (PM group) or they took placebo for 12 weeks.
Results: No significant difference in sleep quality, as assessed by the Pittsburgh Sleep Quality Index (PSQI). However, 5-times sit-to-stand (5-STS) and drowsiness were significantly improved in the PM NMN group, which the authors state “suggests that NMN intake in the afternoon is more effective in improving lower limb function and reducing drowsiness in older adults.”
NCT04823260: (Completed September 9, 2021). Title: A MultiCenter Two Part Study to Evaluate the Efficacy and Safety of NMN as an Anti-ageing Supplement in Middle Aged and Older (40-65 Years) Adults
Part 1: Randomized, double blind, parallel design, placebo-controlled dose ranging, 6 arm study (subjects took 300 mg, 600 mg, and 900 mg of NMN or rice flour placebo daily for 2 months).
Part 2: A group of subjects took 900 mg of NMN daily for 2 months
Update: Results now available: https://doi.org/10.1007/s11357-022-00705-1 Major Findings:
NMN increases blood NAD concentrations and is safe and well tolerated with oral dosing up to 900 mg NMN daily..Blood NAD statistically significantly increased in all NMN-treated groups at day 30 and day 60 when compared to both placebo and baseline (all p ≤ 0.001), but were highest in 600mg and 900mg groups.
6 min walking test: Statistically significant increase in the 300 mg, 600 mg, and 900 mg groups compared to placebo at both days 30 and 60 (all p < 0.01), with longest walking distances measured in the 600 mg and 900 mg groups
Blood biological age (Aging.Ai 3.0 calculator) increased significantly in the placebo group, but stayed unchanged in all NMN-treated groups at day 60; significant difference between the treated groups and placebo (all p < 0.05)
HOMA-IR: No significant difference
Change in SF-36 scores at day 30 and day 60 indicated statistically significantly better health of all three treated groups when compared to the placebo group (p < 0.05), except for the SF-36 score change in the 300 mg group at day 30.
Authors’ Conclusion: “Clinical efficacy expressed by blood NAD concentration and physical performance reaches highest at a dose of 600 mg daily oral intake.”
NCT04228640: (Completed March 30, 2021). Title: A Multicentre, Randomized, Double Blind, Parallel Design, Placebo Controlled Study to Evaluate the Efficacy and Safety of Uthever (NMN), an Orally Administered Supplementation in Middle Aged and Older Adults.
300 mg of NMN or placebo were taken by adults (45-60 years old) daily for 2 months.
See results of study here. Summary of results: NMN raised serum NAD+ levels, as measured at 30 days and 60 days, however the increase in NAD+ levels was not statistically significant compared to placebo (which also increased NAD+ levels, but to a lesser degree). All other findings were also not statistically significant (6 minute walking test, wellbeing questionnaire, blood pressure, and HOMA IR index which measures insulin resistance).
Authors suggest that a greater dose of NMN may be needed to significantly raise NAD+ levels over placebo.
Active, Not Recruiting Clinical Trials:
NCT04862338: “Pharmacodynamics and Tolerance of Nicotinamide Mononucleotide (NMN, 400mg/Day) in Healthy Adults.”
Summary: … to evaluate the physiological and/or biological actions of nicotinamide mononucleotide (NMN-C) in healthy adults receiving a repeated-dose over the course of 28 days by studying the tolerance and pharmacodynamics of this product.
NCT04910061: “Safety and Pharmacokinetics of Nicotinamide Mononucleotide (NMN) in Healthy Adults.”
Summary: … to investigate the safety, pharmacokinetic profile, and effects of nicotinamide mononucleotide (NMN-C) in healthy adults, 18-65 years of age. The effects will be studied over the course of 30 days in a repeated-dose study through the collection of blood and urine samples, and administration of surveys and questionnaires.
NCT04664361: “Effect of NMN on Muscle Recovery and Physical Capacity in Healthy Volunteers With Moderate Physical Activity”.
Summary: to evaluate the effect of NMN supplementation (250 and 500 mg/day over 38 days) compared to placebo in healthy volunteers with moderate physical activity on muscle recovery, physical capacity, cardiorespiratory recovery, the perception of the arduousness of the effort, the variation in blood lactate levels before and after physical exercise, the perception of the intensity of post-exercise muscle pain (cramps), the body composition and Nicotinamide-Adenine Mononucleotide (NAD+) level in blood.
Currently Recruiting Clinical Trials Accepting Healthy Volunteers:
NCT04571008: “Effect of NMN Supplementation on Organ System Biology (VAN)”
Summary: 16 weeks of supplementation with either NMN (300 mg/day) or placebo in healthy adults 45 to 75 years of age will be used to determine whether the effects on “key cardiovascular and metabolic functions” that have been observed in mice apply to people.
Is there evidence it works in preclinical studies for aging?
Modest improvements in health have been demonstrated in mice. Benefits (in aged mice) of NMN supplements include:
Amelioration of pathological Alzheimer’s pathology (including decreases in cognitive impairment, β-amyloid production, amyloid plaque burden, synaptic loss, and inflammatory responses)[5]
Decreased age-associated detrimental physiological changes (decreased inflammation, suppressed body weight gain, enhanced energy metabolism, improved insulin sensitivity, improved eye function, and more)[9]
Prevented age-associated gene expression changes in a tissue-specific way[9]
New Studies:
Margier et al., 2022: NMN Reduces Mortality Risk and Restores Physical Function Following Chemotherapy[10]
For a list of disease specific benefits of NMN, see table 1 here from Yoshino et al., 2019.
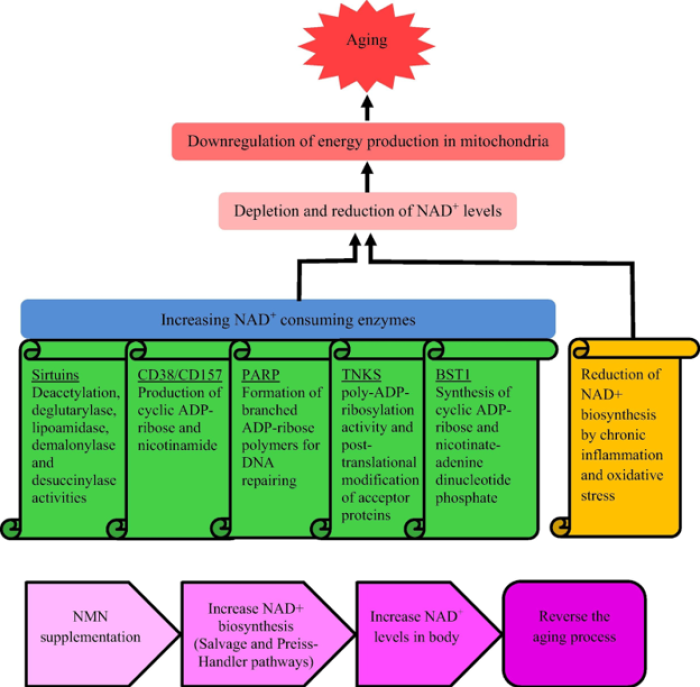
For a full list of studies of NMN for anti-aging in model organisms (including in species other than mice), see table 1 from a 2022 review article by Nadeeshani et al. published in the Journal of Advanced Research. For a depiction of the current understanding of the mechanism by which NMN exerts its effects, see the diagram below from the same review article linked above.
The primary mechanism by which these supplements are thought to mediate these benefits is by raising levels of NAD+, a crucial molecule in cellular metabolism and respiration, levels of which fall naturally with age[11]. Raising NAD+ (NAD+ repletion) is also known to activate sirtuins, which stabilize telomeres[12] and promote healthy aging by signaling nutrient scarcity and catabolism[13]. For a depiction of the mechanism by which NMN is utilized by the cell to produce NAD+, see the diagram below from a 2021 review article by Zapata-Pérez et al. published in the journal EMBO Molecular Medicine:
Are there known safety concerns?
With the exception of a clinical trial that evaluated the safety of a single dose of NMN at either 100 mg, 250, mg or 500 mg, there are no published results on the safety and efficacy of NMN in humans. The aforementioned trial found that a single dose of NMN in the above amounts was “safe and effectively metabolized.” Studies in rats and mice have not found adverse effects of NMN at very high doses, but more, longer-term studies are needed.[8]
Literature Cited: