On May 23, 2024, the Clock Foundation team hosted another Longevity Connect webinar. This latest edition explored the GrimAge Test, its report, and the associated Systems Aging Risk Factors.
GrimAge Test is the epigenetic biological age test used by top longevity researchers and has been taken by thousands to to track their biological age and gain insights into their personalized aging profile. The GrimAge Report provides 10 Systems Aging Risk Factors that influence your biological age and impact your unique biological aging processes.
For those looking to take a proactive, preventive, personalized, and data-driven approach to long-term health and longevity, this webinar offers cutting-edge knowledge to help you understand and influence your aging process, potentially enhancing your lifespan using GrimAge.
At the start of the session, long-time biohacker and Head of Product, Mark Koester, shared insights into his health and longevity journey using biological age tests. Koester shared his own health and aging reports and demonstrated with his GrimAge results how, over several years, he has reduced his biological age to be 6.1 years younger than his chronological age.
Epigenetic expert and Executive Director, Bobby Brooke, then explained the GrimAge Epigenetic Age Test, its mechanisms, and the key physiological systems it assesses. Specifically, he looked at how the different GrimAge System Aging Risk Factors can help paint a picture into specific tissues and physiological systems such as metabolic, inflammatory, brain, and immune systems, among others.
Tracking biological age with GrimAge and its various risk factors enables individuals and groups to decode their unique aging profiles. This collective effort enhances our biological understanding of aging.
Individuals and groups can use GrimAge reports and the biological processes underlying our aging to determine whether their anti-aging strategies and interventions are working. They can also uncover possible opportunities ahead tailored towards unique biological mechanisms that impact aging and GrimAge’s ability to measure it in our epigenetics.
Finally, Brooke provided an overview of the latest and most promising developments in longevity research, epigenetic clocks, and related areas.
Below is the full recap, citations, summary, and screenshots from the webinar. Hope you enjoy!
Agenda and Table of Contents of Topics Covered
- ???? A Personal Journey Tracking and Reversing My Biological Age with Mark Koester
- ???? Unpacking GrimAge and Its System Aging Risk Factors with Bobby Brooke
- ⏰ Review of Epigenetic Clock Data Papers
- ???? / ???? Treatment News ????????⚕️
About Us:
Founded by Steve Horvath and Bobby Brooke, the nonprofit Clock Foundation (https://clockfoundation.org) is dedicated to pushing the boundaries of longevity research for tangible improvements in human health and lifespan. We’ve completed over 300 longevity projects in our first 3 years.
Through MyAgingTests.com, we offer cutting-edge biological age testing and a range of longevity services and software solutions for individuals and groups (clinics, researchers and coaches) interested in quantifying aging and managing anti-aging programs, intervention studies and trials.
Learn more at https://clockfoundation.org/get-started/.
Longevity Connect is a Monthly Longevity Science and Anti-Aging Strategies Virtual Meetup, brought to you by Clock Foundation and MyAgingTests.com.
Got questions or interested in joining our next Longevity Connect event? Check our event schedule and signup at https://myagingtests.com/longevity-connect/.
Disclaimer
Disclaimer: Nearly all aging biomarker tests today are “research use only” tests, which means they are not approved by FDA for use in treatment of any diseases. There is no guarantee that even with the best measures of biological or epigenetic age, i.e. epigenetic clocks, that if you reverse it by 5 years it will always translate into 5 extra years of life.
You should always consult your physician before initiating any new treatment.
Conflicts of Interest: I (Bobby Brooke) work with a private biotech company Intervene Immune developing treatments for immune system regeneration. Through Clock Foundation I work as a non-profit collaborator for dozens of aging researchers, including many academics and longevity biotech companies.
For Longevity Connect recaps, we focus on analysis of data that’s already been published, and largely on treatments that are already available for use in clinical trials.
Key Topics: Longevity Connect on May 23, 2024
A Personal Journey Tracking and Reversing My Biological Age with Mark Koester
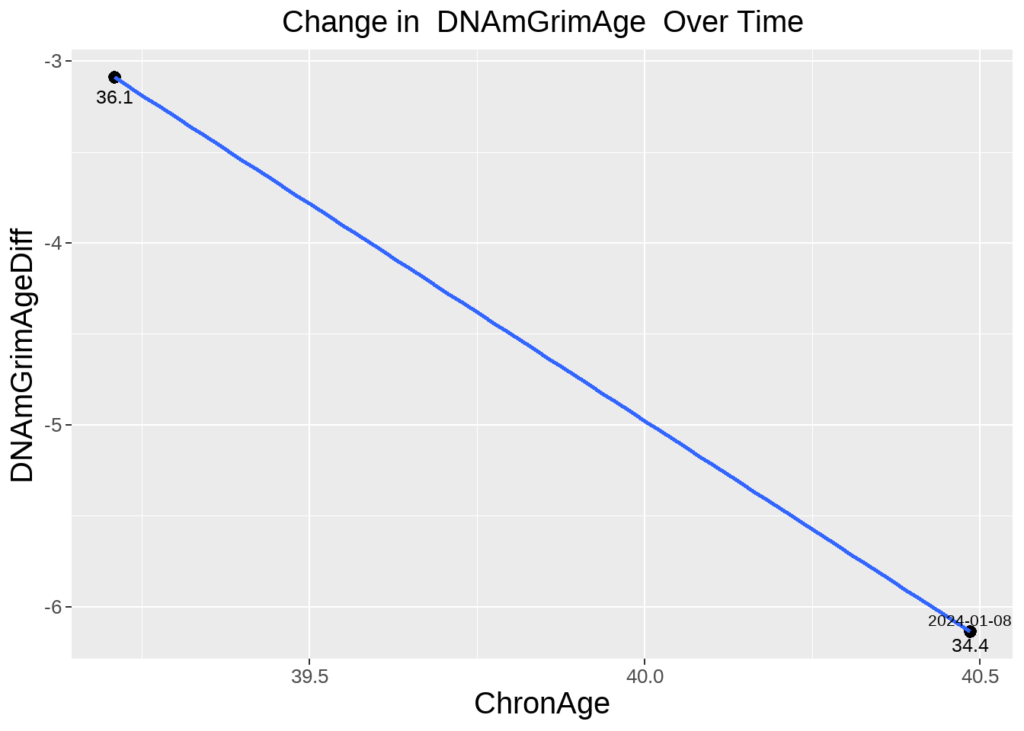
Snapshot of Mark Koester’s Progress in Decreasing Biological Age Between Two Tests
What do GrimAge Test results look like, and how might you use them to guide your longevity journey?
“I’m happy to report that my efforts in sleep, health, and wellness have successfully decreased my rate of aging over time,” said Longevity Biohacker and Head of Product, Mark Koester, to open the webinar.,” said Longevity Biohacker and Head of Product Mark Koester to open up the webinar. He shared his latest biological age test results, demonstrating how, over several years, he has reversed his biological age to be 6.1 years younger than his chronological age.
Here are the results of his first GrimAge epigenetic age test taken in September 2022:
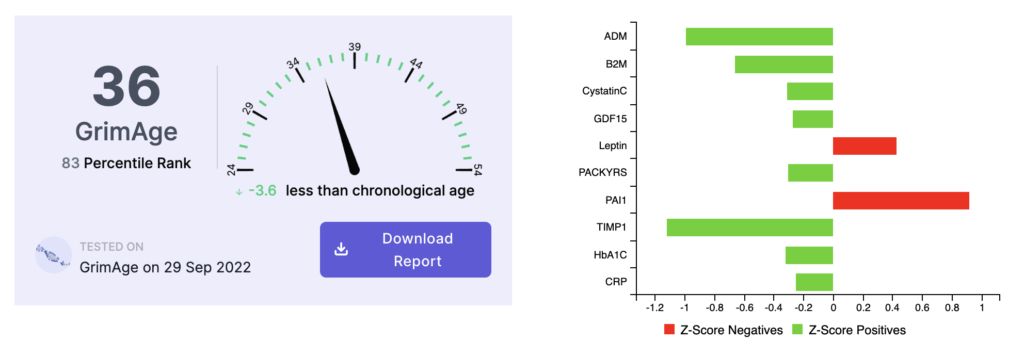
The initial test in September 2022 indicated his biological age was 3.6 years below his chronological age, with the primary age driver identified as PAI1, also known as Vascular Age.
Here is what his second test results looked like:
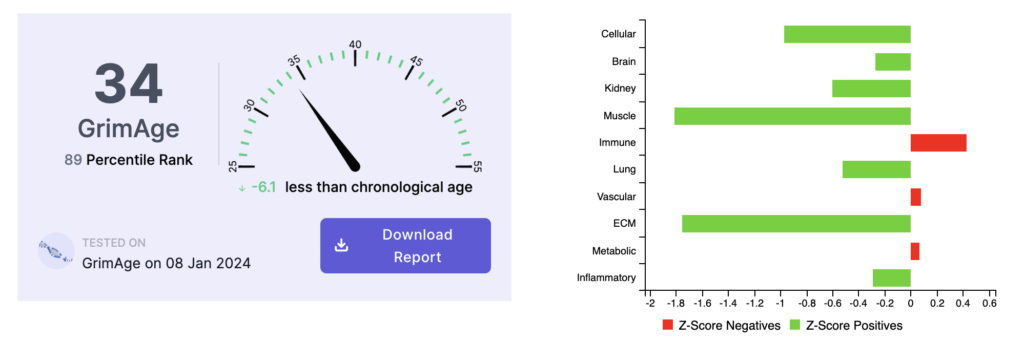
The second test in January 2024 showed his biological age to be 6.1 years below his chronological age. Interestingly, the Vascular Age risk factor had decreased dramatically, while the Immune Age/Leptin risk factor remained his most persistent and notable trending risk factor.
What lifestyle changes did he make between tests, such as improving sleep, managing stress, and exercising more regularly?
Koester noted that everyone ages differently and understanding your unique aging drivers is key to living a longer, healthier life. He shared how his exercise routine and overall health were dramatically impacted by the COVID-19 pandemic and lockdown, leading to an increased biological age and worsening blood biomarkers. Through a series of lifestyle changes and test/retest periods, he managed to improve both his lifestyle and his biological age.
Specifically, he emphasized how regular exercise (particularly running), improved sleep (tracked with Oura), and stress management helped decrease his biological age. He also mentioned that switching to a plant-based diet and incorporating intermittent fasting played significant roles.
Specifically, he talked about how exercise (specifically running), sleep (tracked with Oura) and stress management all helped him personally decrease his biological age. He talked about switching to a plant-based diet and intermittent fasting have also played a role.
He also commented that work and life stressors are now better managed and humorously noted, “Bobby’s not overstressing me… And I’m happy to say we’re making progress both in business and my own health journey.”
Koester demonstrated that biological age can be altered through simple lifestyle changes and test/retest periods. He argues that lifestyle modifications such as diet, exercise, sleep, and stress management should be the first line of action when aiming to reverse biological age.
Which risk factors did he identify as having the most opportunity for improvement based on his GrimAge reports?
Koester highlighted that his Leptin/Immune Age risk factor remains his biggest opportunity for targeted interventions:
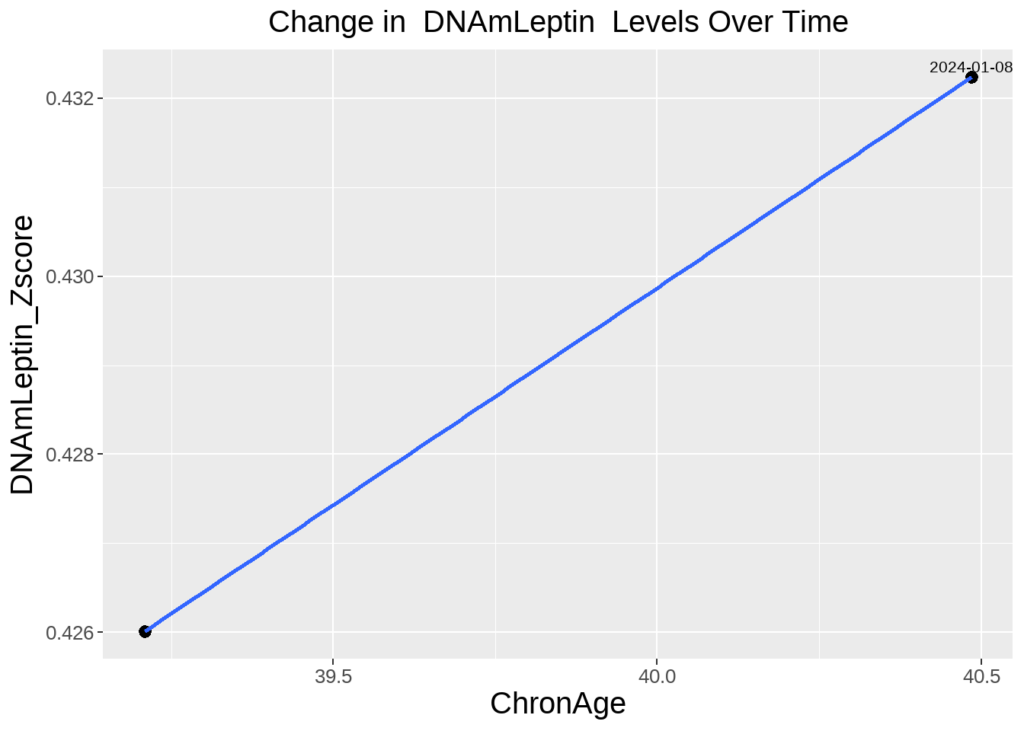
A persistent risk factor identified in multiple tests is a strong indicator of one’s unique aging profile. In Koester’s case, Leptin/Immune Age presents a significant candidate for specific anti-aging interventions.
Koester also shared his longitudinal tracking report from MyAgingTests.com, which is based on multiple tests and data points. This report helps users see changes over time, understand treatment effects, and focus on unique changes. His report highlighted specific changes in his epigenetic clocks and individual risk factors.
He previewed new data collected from wearables on HRV age, sleep, and activity. Many people in our community use a variety of wearables, and we’re excited to leverage these digital health metrics to understand how lifestyle, sleep, and stress impact biological age and epigenetics.
Finally, he noted his excitement about participating in one of our Longevity Groups. These group initiatives involve members following the same protocol and sharing anonymized age test results to determine effective, evidence-based anti-aging interventions for everyone.
Unpacking GrimAge and Its System Aging Risk Factors with Bobby Brooke
“At the Clock Foundation, our goal is to figure out the aging process,” noted epigenetic expert and Executive Director Bobby Brooke as he opened his in-depth dive into GrimAge and its System Aging Risk Factors.
GrimAge is a cutting-edge biological age test that provides unique, independent, but interconnected aging risk factors, which can be mapped to metabolic, inflammatory, and other physiological biological systems.
By tracking biological age with GrimAge and its various risk factors, individuals and groups can start to decode their unique aging profiles and determine whether their anti-aging strategies and interventions are working.
Understanding the GrimAge Epigenetic Age Test
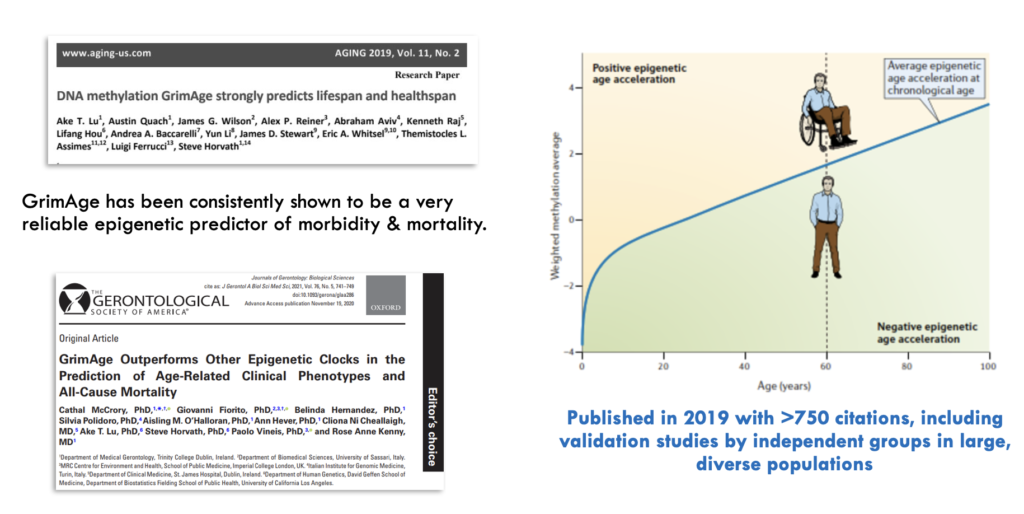
GrimAge is a biological age test developed by researchers, including Steve Horvath, one of the founders of the Clock Foundation. It is an epigenetic predictor of human healthspan and lifespan and has been taken by thousands of people. GrimAge is one of the most widely validated epigenetic clocks, cited in over 700 studies.
GrimAge analyzes DNA methylation patterns in blood to calculate an overall biological age score.
GrimAge is named after the Grim Reaper, as a higher GrimAge biological age correlates with increased mortality risk and earlier onset of age-related diseases. A lower GrimAge biological age indicates better outcomes in terms of mortality, disease risk, and aging.
In addition to predicting all-cause mortality, GrimAge can predict the onset of various age-related diseases, such as cancer, cardiovascular disease, and cognitive decline. Numerous studies have shown a strong association between accelerated GrimAge and mental health conditions, which is particularly interesting and somewhat surprising for a blood-based biomarker.
Recent data has revealed new links to cognitive outcomes. For instance, some independent studies have shown a robust correlation between accelerated GrimAge and cognitive decline or poor cognitive function.
GrimAge has become a promising tool for a diverse array of screening applications, including all major age-related diseases, immunity, mental health, cognitive decline, and more. For example, GrimAge can predict cancer onset, cardiovascular disease, cognitive aging, kidney disease, fatty liver, respiratory function, and many frailty measures (Lu 2019, Li 2020, Hillary 2020, McCrory 2020, Hillary 2018). It might even provide a measure of trauma.
The GrimAge Test is used by longevity researchers to validate promising longevity therapies and by individuals and groups to measure and monitor their biological aging and related risk factors over time.
Understanding GrimAge Risk Factors
GrimAge is a unique epigenetic clock, originally built from 8 but now consisting of 10 risk factors and components. Each one represents a unique aspect of the aging process or your epigenetic risks for mortality and age-related diseases. While related and showing some correlation, each risk factor is independent, representing and providing different aspects of the aging process.
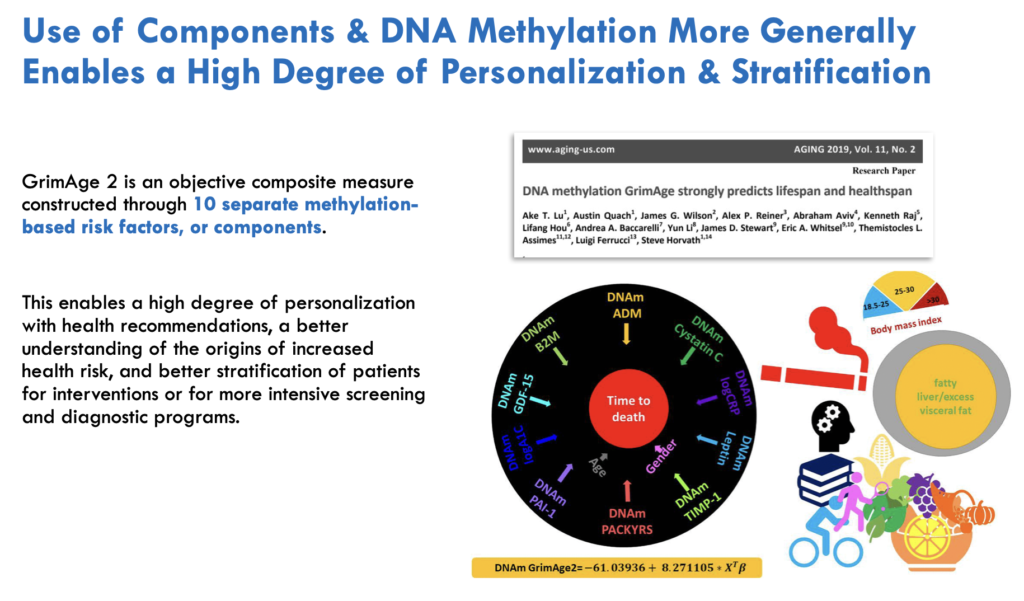
Rather than just providing an overall biological age, GrimAge examines levels of 10 specific aging-related biomarkers or ‘risk factors.’ Understanding these factors offers insights into an individual’s physiological aging processes and potential lifestyle and medical interventions that could reduce their biological age.
GrimAge and its risk factors enable greater personalization and segmentation based on unique aging profiles and shared aging risk factors. By identifying your primary and secondary risk factors, you can develop personalized strategies to reduce your GrimAge and epigenetic age, potentially extending your healthspan and lifespan.
What does each GrimAge risk factor measure at a biological level? What tissues and physiological systems do different risk factors provide insights into?
A GrimAge risk factor generally measures a specific biological marker or process related to aging. Each risk factor assessed by GrimAge corresponds to a different aspect of physiological aging occurring at the tissue, cellular, or molecular level in the body.
By understanding the biological systems behind each of these unique GrimAge risk factors, you can begin to navigate possible interventions and identify the most effective treatments for reducing your risk.
For example, some key risk factors measured by GrimAge include markers of metabolic aging and inflammation, such as HbA1c and CRP. Others reflect processes like cellular senescence (GDF15), kidney function (Cystatin C), neuroinflammation (B2M), and more.
Ultimately, by analyzing multiple risk factors, GrimAge aims to provide a comprehensive view of the rate and patterns of biological aging occurring in individuals, groups, and communities.
Let’s examine the GrimAge risk factors one by one to understand how they help decode the biological aspects of aging.
●
Cystatin C & Kidney Function
The GrimAge risk factor Cystatin C (also sometimes written as “statin”) measures kidney function as it relates to aging.
Cystatin C is a protein produced by all nucleated cells in the body and cleared from the bloodstream by the kidneys. It functions to prevent the breakdown of proteins. Clinically, it functions to prevent the breakdown of proteins and is used as a biomarker for kidney function. When kidney function declines, levels of Cystatin C rise in the bloodstream due to the kidney’s inability to excrete or clear them.
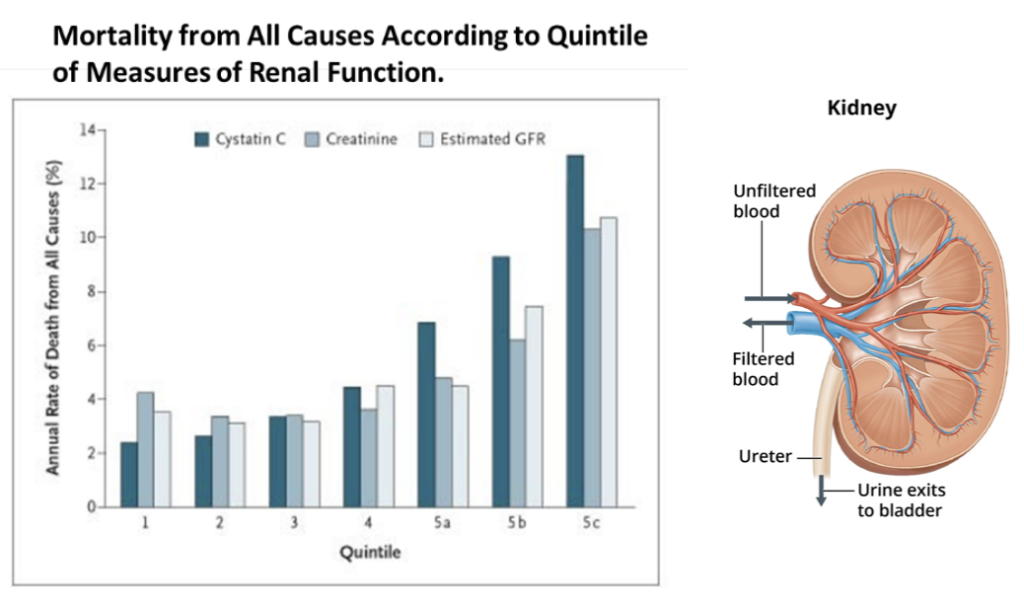
Cystatin C levels naturally increase with age in adults, regardless of clinical kidney disease risk. In GrimAge, the Cystatin C risk factor, based on methylation patterns, acts as a proxy marker of kidney aging. Higher levels suggest reduced kidney clearance ability over time, which can impact overall health and longevity if not managed properly. Understanding an individual’s Cystatin C/statin risk factor profile through GrimAge testing can provide valuable insights into their renal aging processes.
●
GDF-15 & Muscle Function
GDF-15 (Growth Differentiation Factor 15) is a factor related to inflammation and is sometimes referred to as a “Mitokine,” meaning it gets produced in response to mitochondrial stress.
More generally, GDF-15 is a protein marker that is typically present at low, nearly undetectable levels in the adult body but shows higher expression and presence in response to oxidative stress, DNA damage, disease or injury, or other stresses. Higher GDF-15 levels might indicate greater cellular senescence and mitochondrial deterioration.
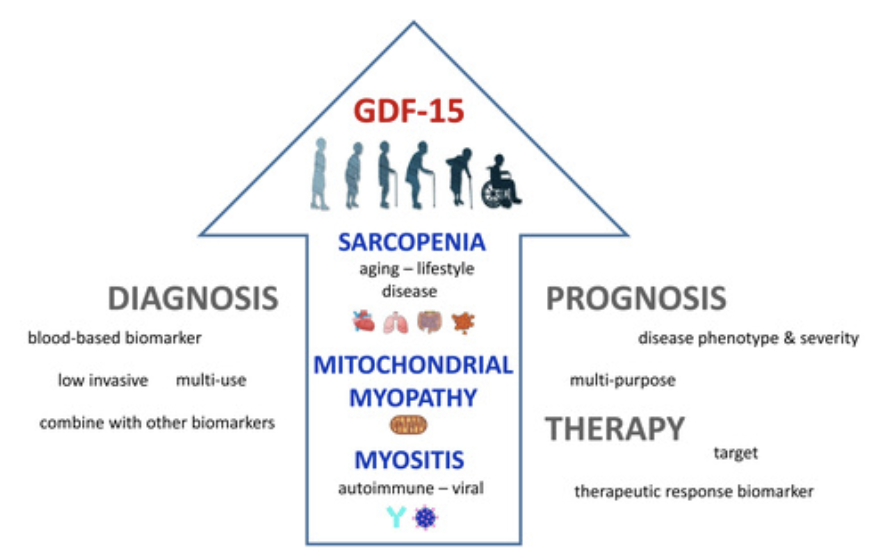
GDF-15 levels moderately increase with and impact cellular senescence. As we age, senescent “zombie-like” cells accumulate and secrete inflammatory molecules that can damage tissues over time. GDF-15 acts as a proxy for this cellular aging process, providing insight into an individual’s muscle health and risk for age-related conditions like sarcopenia.
Elevated levels of GDF-15 are associated with various age-related diseases including inflammation, cancer, cardiovascular diseases, obesity, sarcopenia (age-related muscle wasting), and mitochondrial dysfunction.
Tracking one’s GDF-15 risk factor profile through GrimAge can help understand and target anti-senescence lifestyle strategies as well as provide a proxy to track one’s muscular age.
●
HbA1c and Metabolic Function
Clinical HbA1c (glycated hemoglobin) is a blood biomarker that measures average blood sugar levels over the past 2-3 months. It forms when blood sugar binds to hemoglobin in red blood cells. Higher HbA1c indicates worsening blood sugar management and insulin resistance over time. Since RBCs have a lifespan of about 3 months, the HbA1c test reflects the average blood glucose levels over that period, providing a picture of long-term glucose control. Elevated HbA1c levels have clinical implications and can indicate insulin resistance or other conditions affecting glucose metabolism (like diabetes).
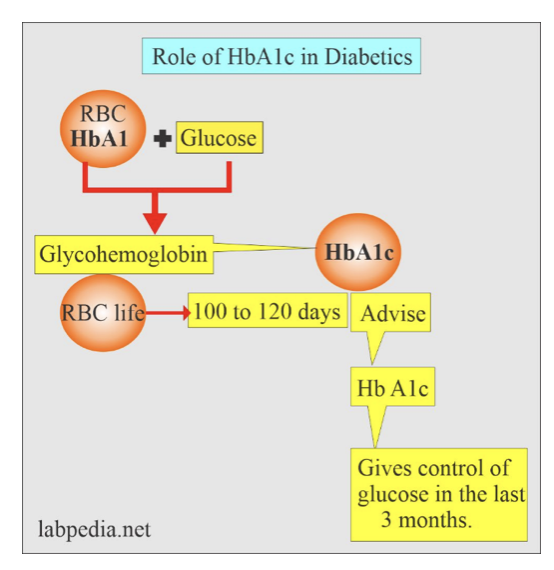
Source: Diabetes in older adults – a consensus report
The GrimAge risk factor HbA1c measures metabolic aging at an epigenetic level. As a risk factor in GrimAge, HbA1c acts as a marker of glycemic health and metabolic function. Elevated levels suggest accelerated aging of tissues susceptible to glucose toxicity, like nerves, blood vessels and organs.
Understanding one’s HbA1c GrimAge risk factor provides insights into lifestyle behaviors like diet, exercise and stress management that impact metabolic aging.
●
CRP and Inflammatory State
CRP (C-reactive protein) is a marker of systemic inflammation throughout the body. It’s a protein produced by the liver in response to inflammation. Specifically, CRP is released into the bloodstream in response to signals from macrophages and fat cells. Elevated CRP levels indicate increased inflammation throughout the body and affects the immune system.
Some of the principal reasons for elevated CRP include acute infection or illness (having a cold or the flu), obesity, poor diet, stress, and lack of exercise. Some individuals may be genetically predisposed to higher CRP based on differences in their inflammatory gene expression.
As we age, chronic low-grade inflammation tends to rise and contributes to age-related diseases. In fact, CRP acts as a marker of this aging-associated inflammaging process.
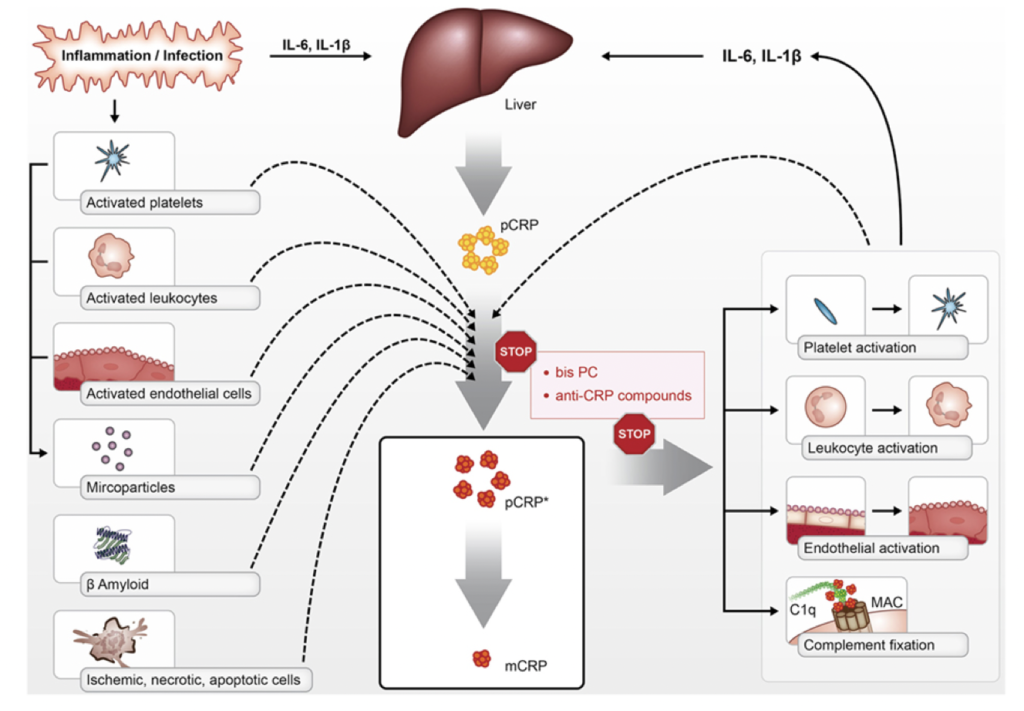
Source: Inflammageing – chronic inflammation in ageing, cardiovascular disease, and frailty
The GrimAge risk factor CRP (C-reactive protein) measures systemic inflammation in our epigenetic markers. Higher CRP as a risk factor in GrimAge suggests a pro-inflammatory biological state that could accelerate cellular damage over time if not mitigated through lifestyle modifications like stress management and nutrition. Understanding an individual’s CRP risk profile provides insight into their inflammatory aging processes.
●
PACKYRS and Lung Function
The GrimAge risk factor PACKYRS tracks your lung function.
Unlike the other risk factors that are based on proteomic markers or predictors of other aging biomarkers, PACKYRS (which stands for number of packs smoked over years) is a DNA methylation-based surrogate for an individual’s smoking exposure and history over their lifetime. Smoking presents a well-defined model of accelerated lung aging. If somebody has smoked a pack a day for 10 years, that has a big effect on DNA methylation and accelerates epigenetic aging, which is captured and quantified by this PACKYRS component.
While PACKYRS directly reflects tobacco use, it also acts as a predictor of lung aging and respiratory function even in non-smokers. This suggests smoking, environmental toxins, air pollution, and other factors accelerate biological processes related to lung health. In fact, studies have shown air pollution impacts epigenetic age, though nowhere to the extent as cigarette smoking.
Understanding an individual’s PACKYRS risk factor can provide insight into their pulmonary aging trajectory and associated health risks.
●
PAI-1 and Vascular Function
The GrimAge risk factor PAI-1 measures blood clotting and is indicative of vascular function and vascular aging.
PAI-1 (plasminogen activator inhibitor 1) is a protein with an important physiological role. It is involved in the regulation of (fibrin) clot formation and its breakdown. Specifically, higher levels of PAI-1 inhibit the breakdown of clots.For example, if you’re wounded, PAI-1 is recruited to the site and ensures there’s no excessive blood loss. As you get older, PAI-1 levels appear to become elevated too.
PAI-1: The Fountain of Youth Molecule in the Amish Community: Doug Vons and a team of researchers at Northwestern University have discovered a remarkable phenomenon in certain Amish communities. These communities exhibit a genetic condition known as PAI-1 (plasminogen activator inhibitor-1) knockout, resulting in minimal expression of this molecule. As a consequence, individuals in these communities tend to live 10 to 20% longer than average and display impressive cardiovascular health well into old age.
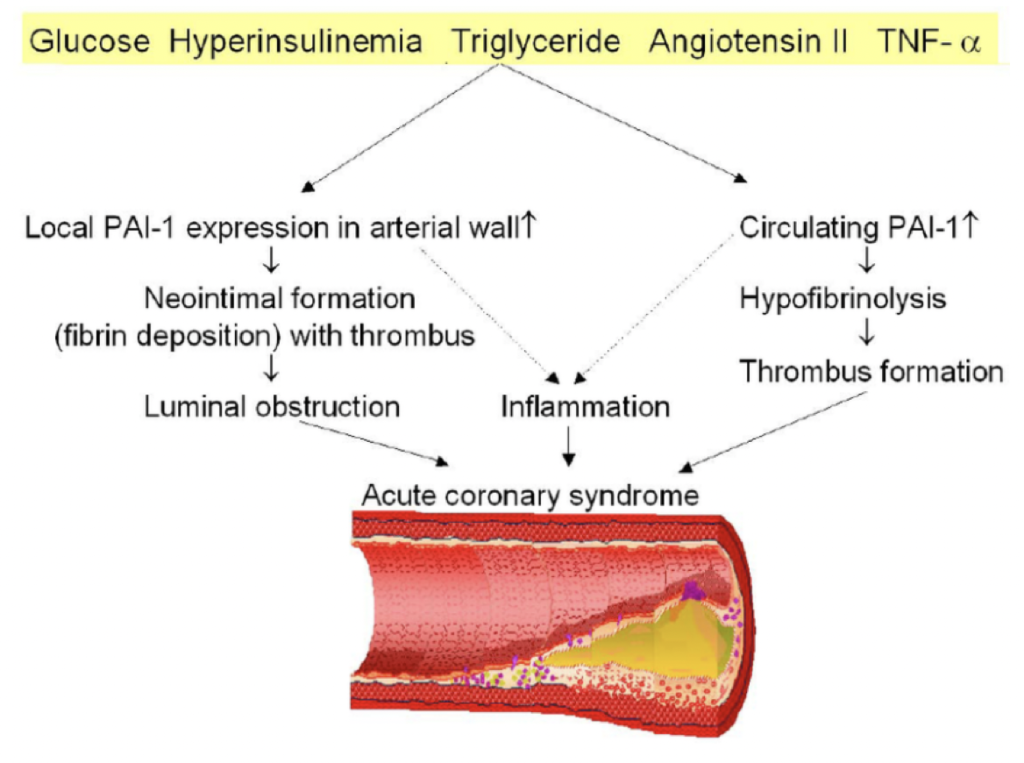
Source: Effects of Altered Plasminogen Activator Inhibitor-1 Expression on Cardiovascular Disease
Despite the lack of PAI-1, these Amish individuals maintain adequate blood clotting abilities, suggesting that other physiological factors compensate for the deficiency. This unique genetic trait drastically reduces their risk of cardiovascular disease, highlighting the potential of PAI-1 as a key target for longevity research and leading some to refer to it as a “fountain of youth” molecule.
●
TIMP-1 and Extracellular Matrix Function
TEMP-1, or Tissue Inhibitor of Metalloproteinases-1, plays a crucial role in regulating the extracellular matrix by inhibiting enzymes called metalloproteinases (MMPs) that degrade and remove the extracellular matrix of cells. Elevated levels of TIMP-1 can indicate endothelial dysfunction and a loss of tissue barrier integrity. This protein helps prevent the destruction of cells and tissues, thereby promoting cell proliferation and growth in various biological processes such as bone remodeling, blood vessel formation, wound healing, fibrosis (scarring), and inflammation.
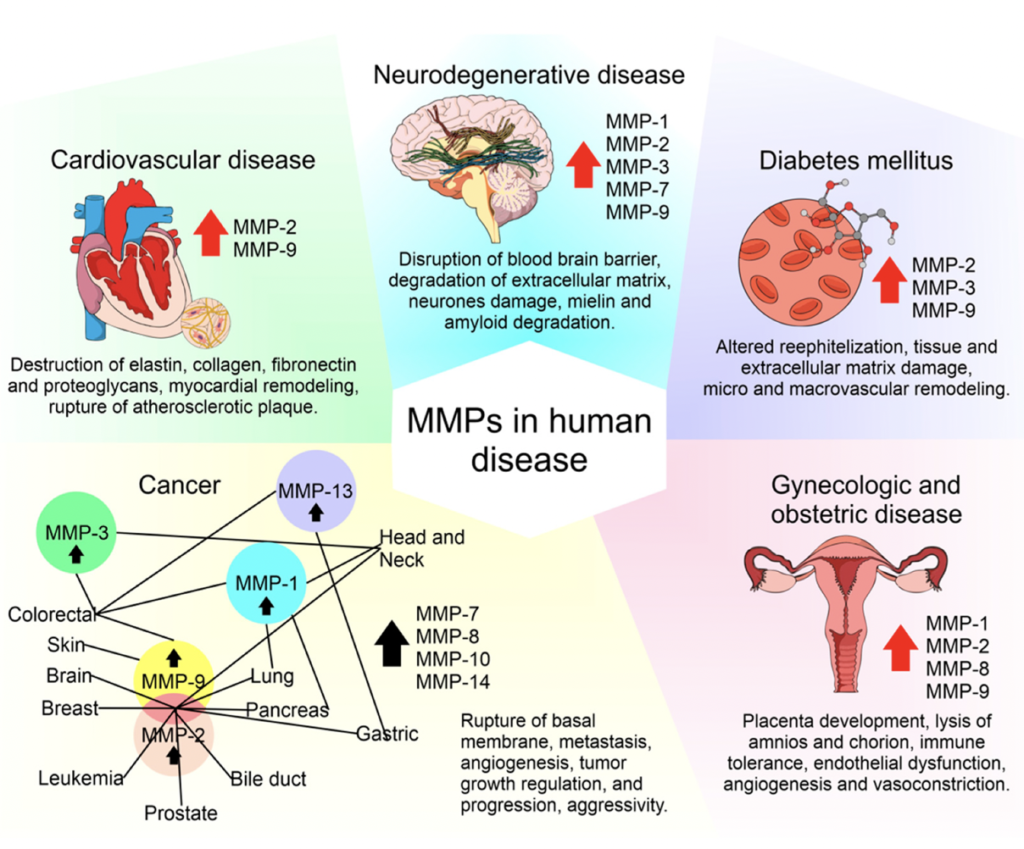
Source: The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases
TIMP-1 also appears to be involved in maintaining barrier integrity in different tissues, including the blood-brain barrier and the intestinal barrier. However, its role can become problematic with age. In older individuals, elevated TIMP-1 levels are associated with increased fibrosis, reduced tissue rejuvenation, and greater frailty, reflecting a more fibrotic state common in aging tissues.
As a GrimAge risk factor, TIMP-1 reflects age-related increases in fibrosis and declines in tissue rejuvenation capacity. Understanding an individual’s TIMP-1 profile can provide valuable insights into vascular health, blood-brain barrier integrity, and susceptibility to frailty in old age.
Since it’s still rather esoteric, further research is needed to fully elucidate the role of TIMP-1 and how it can be targeted for therapeutic intervention.
●
Leptin & Immune System Modulation
Leptin is known as the “satiety” hormone. It is produced by fat cells and secreted into the bloodstream when fat stores increase and helps regulate appetite and metabolism. It also believed to play a role in modulating immune cell function. Besides regulating hunger, leptin plays roles in reproduction, blood vessel formation, bone maintenance, wound healing, and glucose metabolism. Similar to insulin resistance, leptin resistance occurs when higher levels of leptin in the bloodstream do not effectively perform their regulatory functions.
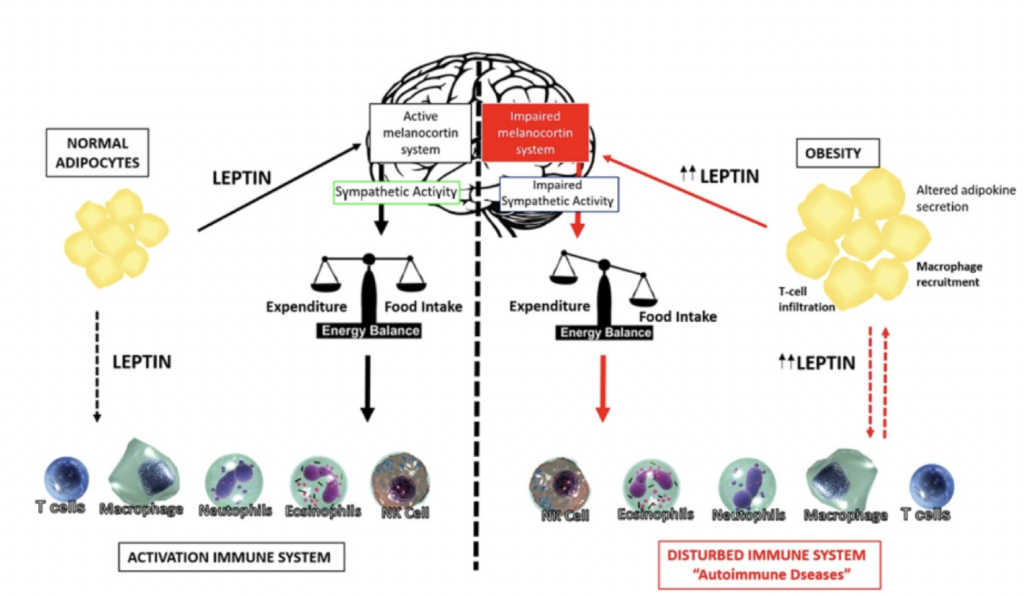
Source: Leptin as a novel therapeutic target for immune intervention
While Longitudinal GrimAge reports provide other measures of immune function, the GrimAge risk factor Leptin can also help you measure immune system function and immune age based on epigenetic markers.
As a GrimAge risk factor, elevated Leptin levels suggest chronic overactivation of inflammatory immune responses with aging. This pro-inflammatory state may contribute to immunosenescence, a weakening of immune defenses over time. Understanding an individual’s Leptin risk profile can provide insight into how well their immune system is able to maintain homeostasis and fight infection and disease as they age.
●
ADM & Cellular Aging
Adrenomedullin (ADM) is a peptide hormone initially discovered in a tumor of the adrenal gland. It circulates in the blood and is expressed in virtually all human tissues. ADM plays several critical roles in the body, including vasodilation (widening of blood vessels), regulation of hormone release, promotion of angiogenesis (creation of new blood vessels), and antibacterial immune activity.
ADM is also significant in the context of disease. It is involved in tumor progression and may serve as a biomarker for cardiovascular diseases. Elevated levels of ADM are observed in individuals with hypertension and heart failure, indicating its potential role in these conditions.
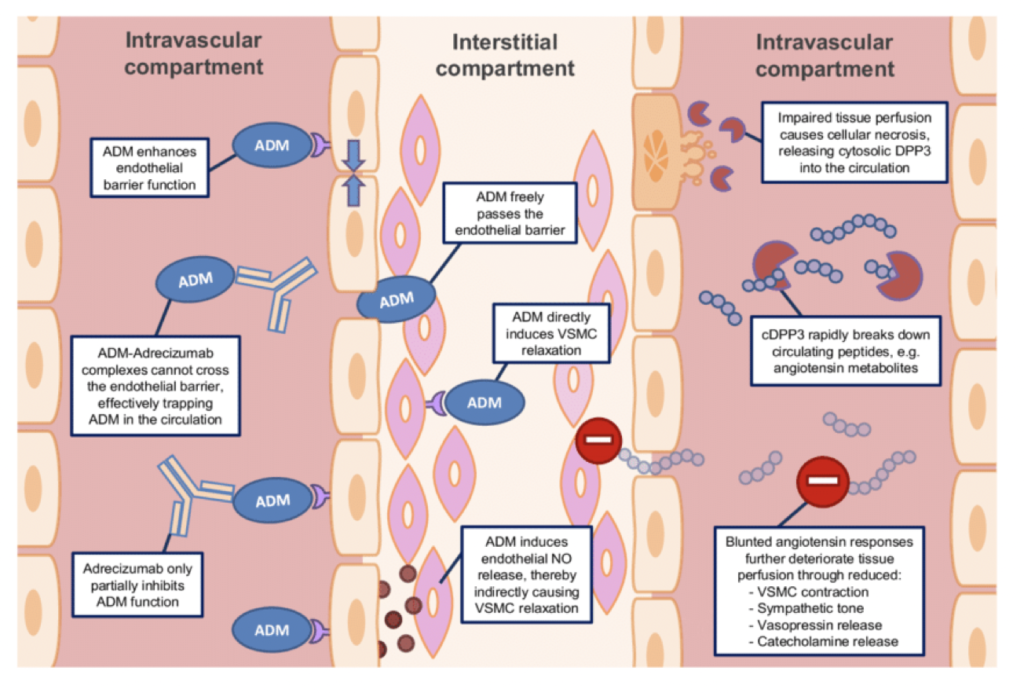
As a GrimAge risk factor, ADM measures vascular function and endothelial cell health at a biological level. Its regulatory functions extend to blood pressure and endothelial cell growth, and it also acts as an antioxidant and anti-inflammatory agent. Elevated ADM levels may reflect vascular aging processes, such as endothelial dysfunction and oxidative stress. However, its precise role in aging is still being researched.
Understanding an individual’s ADM risk factor could provide insights into their vascular health and potential age-related cancers. However, more research is needed to fully elucidate the physiological implications of this biomarker and its relationship to the aging process.
●
B2M & Brain Inflammation
B2M (beta-2 microglobulin) is a protein fragment found on the surface of all nucleated cells and has been specifically linked to brain inflammation. Elevated B2M levels in the blood suggest increased cellular turnover related to immune system activity. B2M tracks immune infiltration, an immune response that typically doesn’t occur in youth due to better blood-brain barrier integrity. Chronic inflammation is a hallmark of aging and can be seen in the increased levels of B2M.
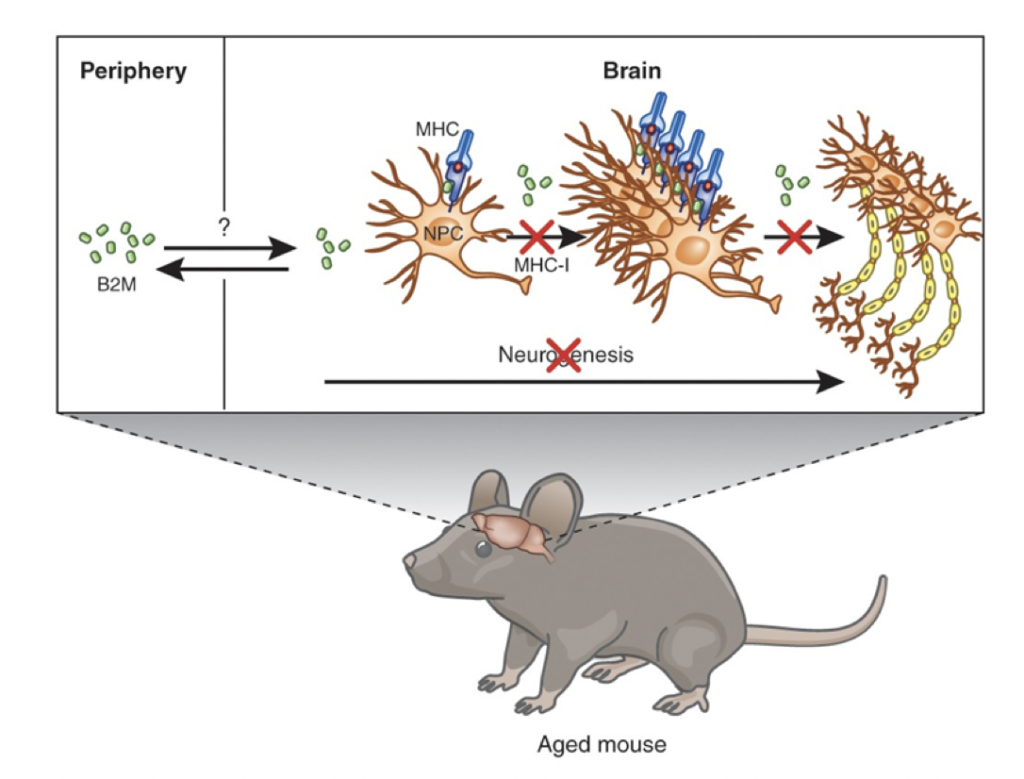
Source: β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis
he GrimAge risk factor B2M measures immune system aging and neuroinflammation using DNA methylation patterns. Higher B2M levels indicate chronic low-grade inflammation and immune system dysregulation associated with aging. This may also reflect age-related neuroinflammatory processes, such as increased microglial activation in the brain. In youth, better blood-brain barrier integrity helps prevent chronic inflammation, but as we age, the integrity of this barrier weakens, leading to more neuroinflammatory states.
Intriguing research involving heterochronic parabiosis, where a young mouse is attached to an older mouse to share a circulatory system, has shown that youth factors from the young mouse can rejuvenate the older mouse. Conversely, factors from the older mouse can age the younger mouse. B2M was identified as a circulating factor in older mice that caused cognitive dysfunction and impaired neurogenesis in the younger mice, highlighting B2M’s role in age-related cognitive decline and neuroinflammation.
Although research into B2M is just beginning, understanding your B2M GrimAge risk factor might provide insights into how well your immune defenses and blood-brain barrier integrity are holding up with age.
Interconnection of Aging Systems: Correlation of GrimAge Risk Factors
While GrimAge was developed to identify unique and independent risk factors of aging and age-related diseases, there are correlations between several factors. This suggests underlying interconnections in the aging process and potential for anti-aging treatments targeting multiple risk factors and risk factor clusters.
Below is the correlation matrix illustrating the relationships between different GrimAge risk factors:
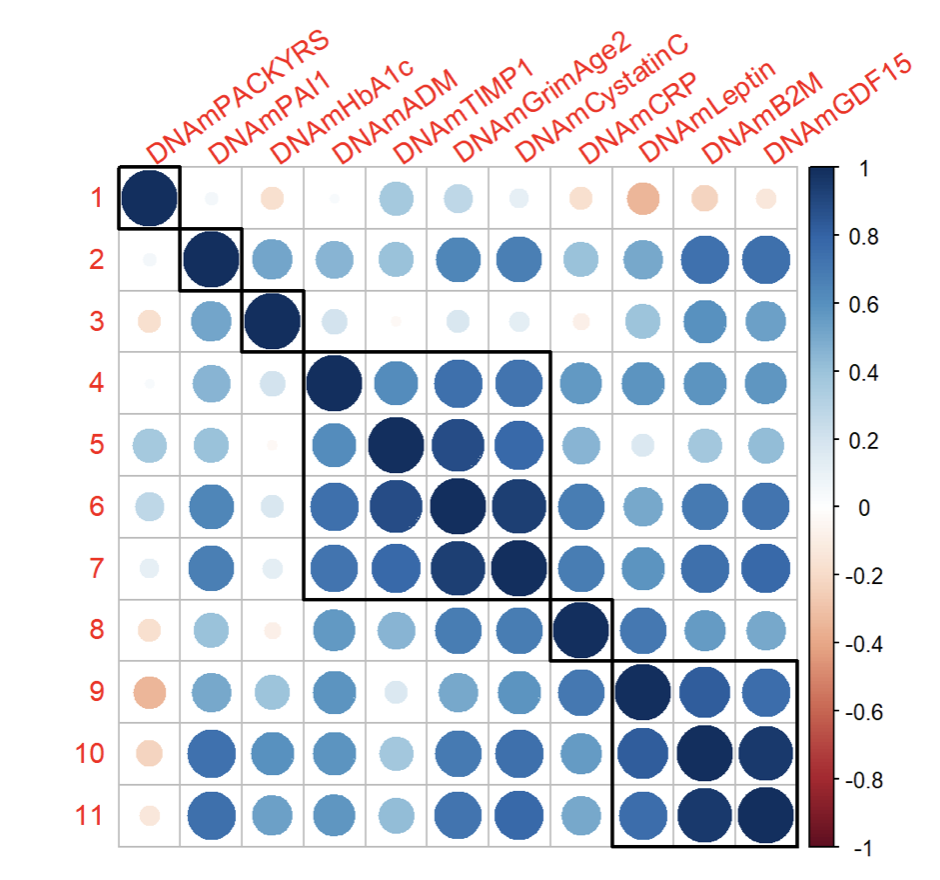
The correlation matrix reveals a few notable clusters:
- Cluster 1: PAI1, HbA1c, CRP, PACKYRS
- Group 1: GrimAge2, ADM, TIMP1, Cystatin C
- Group 2: GDF15, B2M, Leptin
●
“Decoding Aging” Question: How does GrimAge and its risk factors help us better understand aging and age-related diseases?
GrimAge and its risk factors help us better understand aging and age-related diseases in a few key ways:
- Insights in Aging Diseases: Large scientific validation studies have shown that GrimAge risk factor levels strongly correlate with the risk of age-related diseases such as cancer, cardiovascular disease, and neurodegenerative conditions.
- A More Complete Picture of Aging: GrimAge risk factors offer a more comprehensive view of the biological processes underlying aging at the molecular, cellular, tissue, and organ levels. Beyond providing a single biological age score, GrimAge factors can predict and help determine personalized aging processes.
- Specific aging-related mechanisms: Each risk factor offers insights into a specific aging-related mechanism or pathway, such as inflammation, metabolism, cellular senescence, etc. which we hopes to impact mechanism- or pathway-specific candidate interventions.
- Personalized physiological aging profile: By analyzing multiple risk factors together, we obtain a more complete picture of an individual’s overall physiological aging profile and any imbalances.
- Longitudinal tracking benefits: Longitudinal tracking with GrimAge can help determine the impact of interventions on biological aging and potentially delay the onset of age-related diseases.
- Identifying Anti-Aging Interventions: Understanding risk factor levels helps identify potential lifestyle and medical interventions to target specific aging processes. Our GrimAge report provides personalized recommendations based on your primary aging drivers.
Monthly Longevity Research Roundup
With ten of more relevant and interesting longevity papers coming out daily, Bobby Brooke reviewed a few of most notable recent papers on treatments, epigenetic clock technologies and aging mechanisms and more.
⏰ Epigenetic Clock Papers – Clock Data / Results
Identifying the relation between food groups and biological ageing: a data-driven approach
This paper came out looking at different food groups and aging. It was a relatively straightforward study involving a diet survey and looking at whether or not certain food groups are reducing epigenetic aging. Overall, the findings and recommendations were rather conventional and offered only modest effect sizes.
●
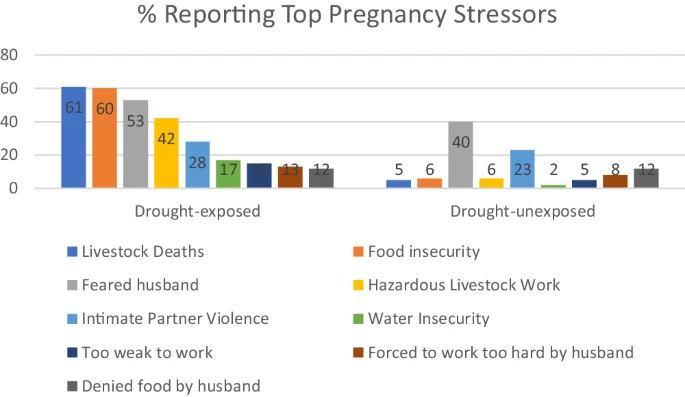
- An intriguing study that looked at mothers who had severe drought exposure and were under stressed. This study showed a clear effect on epigenetic aging, namely accelerating GrimAge and another clock as well as shortened telomere length.
●
Epigenetic age oscillates during the day
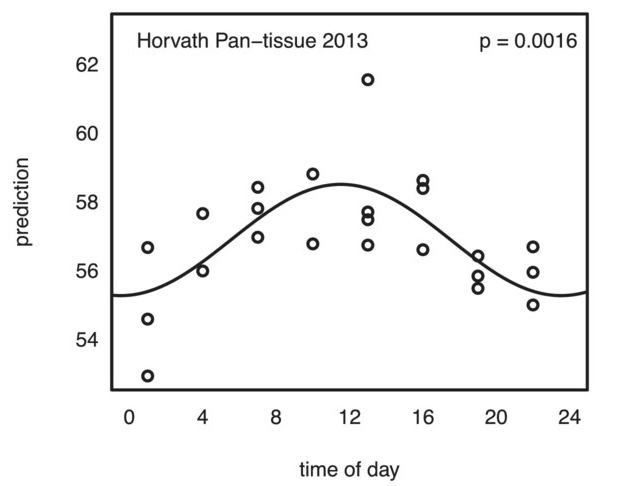
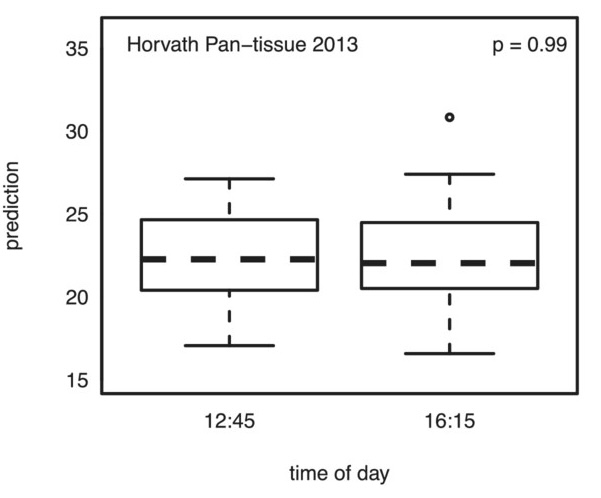
- This study examined how epigenetic clocks change over the course of a day. In this study, a single person was tested over ten times at different times of the day to see if the timing impacted epigenetic clock measures. The findings suggest that the time of day does affect these measures to a modest degree.
- The key takeaway is that when conducting studies or clinical trials on longevity drugs or supplements, it’s important to take epigenetic tests at the same time of day and under similar conditions, such as having the same meal the night before. This consistency helps ensure more accurate results in determining the effects of treatments on epigenetic clocks. Keeping a detailed journal of testing conditions can also aid in achieving reliable outcomes.
Related: Measuring technical variability in Illumina DNA methylation microarrays
●
Tracking single-cell evolution using clock-like chromatin accessibility loci
- This study investigates methylation in single cells. This research highlights the current limitation of using bulk samples for epigenetic clock data, which provide a composite aging score representing various cell types within a single sample. The study introduces new techniques that assess the aging process in each individual cell within a sample. While this technology is currently expensive, it is anticipated that within the next five to ten years, it may become feasible to track the aging of every single cell in a blood sample or other types of samples, offering a more precise and detailed understanding of cellular aging.
●
Aging clocks based on accumulating stochastic variation
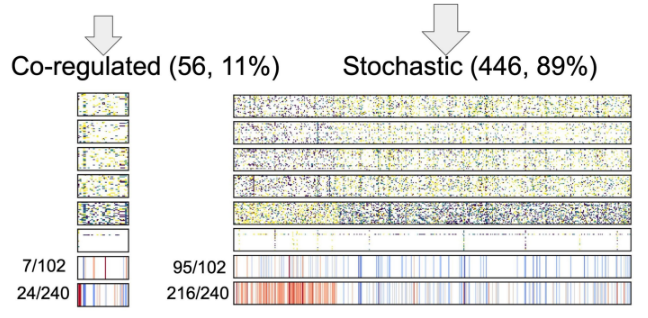
- Is aging programmed vs. wear & tear, or stochastic? Are methylation changes causal vs. downstream? How modifiable is epigenetic aging?
- This paper delves into the debate of whether aging is programmed or the result of wear and tear, and whether methylation changes are causal or downstream effects. The research investigates the genes involved in various methylation sites and aims to determine how much of aging is due to stochastic noise—random disruptions to the epigenetic maintenance system—versus programmed processes where the body intentionally shuts down certain functions over time. The findings suggest that a significant portion of age-related changes are indeed stochastic, reflecting a failure in the epigenetic maintenance system. However, there is also evidence of a co-regulated, potentially programmed aspect of aging. This study raises important questions about the causality of methylation changes and the extent to which epigenetic aging can be modified, highlighting the need for further research to better understand and target these processes.
???? / ???? Treatment News ????????⚕️
Ketogenic diet induces p53-dependent cellular senescence in multiple organs
- A recent preclinical study titled “Ketogenic diet induces p53-dependent cellular senescence in multiple organs” investigated the effects of a ketogenic diet on mice. The study found that a ketogenic diet robustly induces cellular senescence, particularly in muscle tissue, leading to the creation of so-called “zombie cells.”
- While this is generally considered an unfavorable effect, the good news is that it appears to be a short-term phenomenon that wears off over time. This suggests that a ketogenic diet, which has been shown to be beneficial in various settings, may induce a hormesis effect—where low-level damage triggers a repair response that ultimately improves function. Thus, while the ketogenic diet has some strange effects, its overall impact may still be positive in the long term.
●
Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity
- A recent study led by Herb Wiseman’s lab at Stanford, explores an innovative approach to rejuvenating aged immune systems. Using an antibody-based treatment, the researchers cleared out myeloid-biased hematopoietic stem cells, which are associated with the innate immune system. This process created space for the more targeted adaptive immune system, particularly enhancing T cell responses.
- The results demonstrated a significant beneficial effect, suggesting a promising method for improving immune function in older individuals. This work builds on similar research conducted by Wiseman’s lab over a decade ago, highlighting the potential of targeted immune system interventions.
●
- Conducted by Juan Carlos Belmonte’s lab, this study explores metabolite reprogramming to enhance muscle regeneration. The researchers focused on myoblasts, the precursor cells that differentiate into muscle cells, and investigated the metabolic environment conducive to this differentiation.
- They identified a group of metabolites—methionine, glycine, putrescine, cysteine, and S-adenosylmethionine (SAM)—that promote muscle cell generation.
- Two formulations, MIM-6 (containing all five agents) and MIM-4 (excluding cysteine and SAM due to neurotoxicity concerns), were tested in a preclinical model.
- The results showed that these metabolite cocktails had modest beneficial effects on muscle function. This intriguing study suggests that recreating the metabolic milieu of myoblast differentiation can potentially improve muscle regeneration, offering a new avenue for therapies aimed at muscle repair and rejuvenation.
●
- This study involves a reanalysis of data from the National Institute on Aging’s Interventions Testing Program (ITP). The ITP tests various compounds in mice to evaluate their effects on lifespan. This reanalysis identified several additional agents, such as Metformin and green tea extract, that demonstrated a lifespan benefit.
- The survival curves indicate a modest improvement, likely enhancing healthspan or median lifespan rather than maximal lifespan. The findings suggest that these compounds may contribute to better overall health in aging but have limited effects on extending the absolute maximum lifespan.
●
- Two recent studies highlight the potential of extracellular vesicles (EVs), specifically exosomes, in combating age-related declines by improving mitochondrial function and energy metabolism.
- One study explores how EVs from hypoxia-treated 3D-grown gingiva-derived mesenchymal stem cells (GMSCs) can reverse mitochondrial dysfunction in aging GMSCs. This innovative approach demonstrates the potential of EVs to restore cellular function in aging cells.
- Another study investigates the effects of EVs derived from young plasma. These EVs were shown to significantly improve mitochondrial energy metabolism, leading to reduced frailty and enhanced lifespan in aged rodents.
- Exosomes are cell-derived membrane-surrounded vesicles that carry bioactive molecules and deliver them to recipient cells. They are often secreted by young stem cells, including those derived from umbilical cord blood. When isolated and injected, these exosomes exhibit profound benefits, as evidenced by recent research.
- These promising rodent studies suggest that exosome-based therapies could be a viable option for clinical testing in the near future, offering a new avenue for treating age-related conditions and enhancing longevity.
●
- Study looked at patients with bipolar disorder found a modest reduction of epigenetic age on people taking medication.
●
Effect of an Asian-adapted Mediterranean diet and pentadecanoic acid (C15:0) on fatty liver disease: the TANGO randomized controlled trial
- This study investigates the effects of pentadecanoic acid (C15:0), a compound now marketed as “Fatty 15.” Despite the recent marketing buzz, including relatives reporting increased advertisements, the clinical data available on C15:0 remains modest.
- The study, published late last year, demonstrated that adding C15:0 to the diet led to modest improvements in weight loss, with effects comparable to those achieved by diet alone.
- While current data doesn’t show significant positive outcomes, the interest in this compound suggests potential for further exploration. If interest continues to grow, launching a longevity group focused on C15:0 could provide a clearer understanding of its benefits and efficacy.
Related: Media: Small but mighty: the fatty acid that packs a considerable longevity punch
●
- A study conducted in Shanghai, China, explores the use of umbilical cord-derived mesenchymal stem cells (MSCs) for treating aging frailty. The research involved multiple injections of these stem cells and reported significant benefits in both cognitive function and physical fitness scores.
- Importantly, no serious adverse events were observed, and the minimal side effects reported were comparable to those seen in the placebo group, being transient and not related to the treatment.
- This promising study adds to the growing body of evidence supporting the potential of stem cell therapies for aging and preventative medicine, highlighting the need for further research and development in this field.


