Name: Dasatinib
Class: Drug (selective tyrosine kinase receptor inhibitor that is prescribed for certain types of leukemia)
Alias(es): CAS# 302962-49-8; Trade names: Sprycel; Dasanix
Background: Dasatinib, sold under the brand names Sprycel and Dasanix, is a synthetic medication available by prescription only, that has been FDA approved since 2006[1], and is currently approved for treating chronic Philadelphia chromosome-positive (Ph+) myeloid leukemia in both adults and children[2,3]. Dasatinib has shown specificity for clearing senescent cells of certain types, i.e. preadipocytes (progenitor fat cells that have hormonal, metabolic, and pro-inflammatory functions) both in vitro in human cells and in vivo in mice and rats. There are no results yet for clinical studies involving dasatinib for treating aging in humans, but preclinical studies involving the combination of dasatinib and another senolytic, quercetin, (D+Q) to target a broader range of senescent cells have shown promising results in terms of extending healthspan and lifespan and ameliorating age-related dysfunctions (see preclinical studies section).
Is there evidence it works in humans for aging?
ClinicalTrials.gov ID And Reference | Trial Status | Title | Summary |
Active, not Recruiting | The Safety and Effectiveness of Quercetin and Dasatinib on the Epigenetic Aging Rates in Healthy Individuals | This prospective non-randomized clinical study will involve participants (≥ 40 yrs.old) taking 500mg Quercetin and 50mg Dasatinib oral capsules on Monday, Tuesday, Wednesday (3 days in a row) per month for the duration of 6 mo. It will evaluate the change from baseline in epigenetic age test results (to be obtained via the illumina 850k epic array). | |
Not Yet Recruiting | Pilot Study to Test the Safety and Efficacy of Metformin, Dasatinib, Rapamycin and Nutritional Supplements (Bio-quercetin; Bio-fisetin; Glucosamine; Nicotinamide Riboside; Trans-resveratrol) in Reducing Clinical Measures of Aging in Older Adults | This study is also known as the VIAging Deceleration Trial Using Metformin, Dasatinib, Rapamycin and Nutritional Supplements. It will be accepting healthy volunteers ≥ 65 years old.
This study will involve first taking metformin and gradually increasing the dose. Then dasatinib, bio-quercetin, and bio-fisetin will be added. Then glucosamine, NR, and resveratrol will be added. And lastly rapamycin will be taken, at which point the subjects will continue on this intervention (taking all of these compounds) for 12 months. The study will evaluate: adverse effects of treatments, DEXA scans of visceral adipose tissue, systolic blood pressure, senescent cell levels, glucose control, and DNA methylation via GrimAge. Estimated study completion date is December 2023. |
Is there evidence it works in preclinical studies for aging?
Dasatinib + Quercetin (D+Q) preclinical studies: Benefits of D+Q for aging (as demonstrated in preclinical studies) include reduction of senescent cell burden and inflammatory markers, and improvements in cardiovascular health, gut microbiome, fatty liver disease, bone health and disc degeneration, strength and physical health, and cognitive health. See table below for references and details.
Model | Vivo/Vitro | Outcome | Reference |
Mice and human cells | Vivo/Vitro |
| |
Mice | Vivo | Chronic D+Q (oral) cleared some types of senescent cells, and alleviated vasomotor dysfunction in naturally aged mice and mice with established atherosclerosis via modulation of nitric oxide (NO). D+Q reduced aortic intimal plaque calcification but did not affect plaque fibrosis. | |
Mice (aged) | In Vivo | D+Q treatment significantly reduced intestinal senescence (as measured by markers p16Ink4a and p21Cip1) and inflammation (as measured by markers Cxcl1, Il1β, Il6, Mcp1, and Tnfα), and it altered the gut microbiome composition in aged mice (suggests it may treat age-related dysbiosis). Placebo-controlled. | |
Mice (INK-ATTAC) | Vivo | Found that accumulation of senescent cells promotes accumulation of fat in the liver and steatosis (liver fat build-up). Thus aging is a contributing factor in causing non-alcoholic fatty liver disease (NAFLD). D+Q effectively reduced overall liver steatosis by removing senescent cells. Mechanism: mitochondria in senescent cells inefficiently metabolize fatty acids, but senolytic therapy with D+Q therapeutically addresses this. | |
Mice, human cells | Vivo/Vitro |
| |
Mice C57BL/6 (aged) | In Vivo |
| |
Mice (aged) | Vivo/Vitro | D+Q improved bone forming capacity of bone marrow mesenchymal stem cells (BSMCs) from aged mice both in vitro and in vivo. | |
Mice (C57Bl/6J) | Vivo/Vitro | D+Q injected bi-weekly into young (3mo) and old (20mo) mice following injury resulted in blunted muscle regeneration in young mice, but improved muscle regeneration in old mice. Additionally, D+Q treated old mice had significantly less senescence levels than old controls. In vitro experiments showed that D+Q improved myogenic progenitor cell proliferation. | |
Mice (INK-ATTAC) | Vivo/Vitro | There is an age-dependent increase in p16Ink4a-positive senescent cells and SASP (inflammation) in brains of mice. Aged mice also experience cognitive decline.Study used INK-ATTAC mice, in which a drug (AP20187) can eliminate p16Ink4a-positive senescent cells. Both D+Q (given orally) and the AP20187 drug (via injection) resulted in a decrease in p16Ink4a-positive microglia and SASP factors in the hippocampus brain region of naturally aged mice, and both treatments significantly improved cognitive function. This suggests that brain inflammation and cognitive decline may be successfully treated with systemic senescent-cell clearance via oral D+Q. | |
Mice (male) | Vivo | In vivo: D+Q once per month for 4 months significantly lowered senescence levels (p16ink4a mRNA) in bone and the percentage of senescent osteocytes in aged (20mo old) mice vs controls. Significantly cleared senescent cells in 24mo old treated mice, improved spine and femur bone morphology, and improved femur thickness and strength. Lower osteoclast numbers and bone resorption were observed. | |
Mice (SAMP10) | Vivo/Vitro | In vivo, D+Q (oral) results in improved frailty index, motor and cognitive function, and hippocampal senescent cell levels in SAMP10 (brain-aging model) mice. In vitro, inducing senescence via oxidative stress in differentiated muscle and neuronal cells, then treating with D, Q, or D+Q, resulted in decreased p16Ink4a expression and number of senescent cells (SABG assay) via apoptosis. D+Q treatment was more effective than D or Q alone. | |
Wistar rats | Vivo |
|
Dasatinib Mechanism:
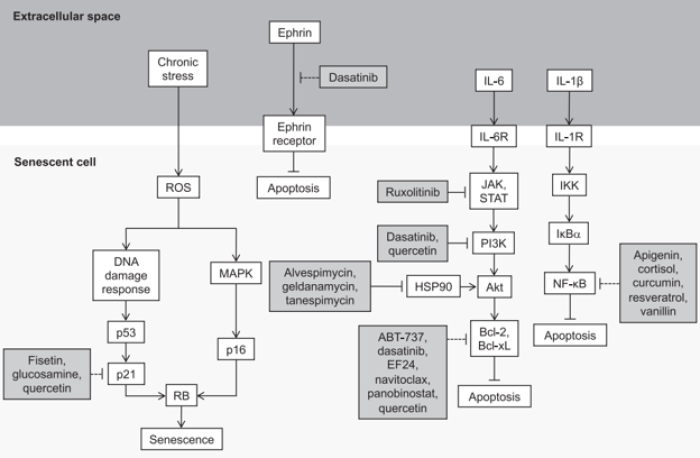
The figure above is from a review by Martel et al., 2020. It shows the pathways inhibited by senolytic supplements, such as Quercetin, and drugs, such as Dasatinib, that lead to reduced viability of senescent cells and/or apoptosis of senescent cells.
Dasatinib (D) is a synthetic src-tyrosine kinase inhibitor, and has been found to be senolytic against human pre-adipocytes without affecting non-senescent cells[4]. D promotes apoptosis of senescent cells by targeting Src family tyrosine kinases, (i.e. BCR/ABL, Src, c-Kit, ephrin), which are known to be Senescent Cell Anti-Apoptotic Pathway (SCAP) components, a pro-survival network utilized by senescent cells to avoid apoptosis[5]. The senolytic activity of D reduces levels of Senescence-Associated Secretory Phenotype (SASP), an inflammatory and damaging property of senescent cells. D is commonly used in combination with quercetin (Q), a flavonoid senolytic that targets senescent endothelial cells, to achieve a broader senolytic effect than when either are used alone[6].
Are there known safety concerns?
In a 2018 human study involving treating idiopathic pulmonary fibrosis (IPF) patients (ages 55-84) with intermittent DQ (D:100 mg/day, Q:1250 mg/day, three-days/week over three-weeks), all patients were retained for the study course with no D+Q discontinuation. Although there was no placebo arm, the adverse events reported were consistent with previous placebo arms in trials for IPF, and there was only one serious adverse event (pneumonia and edema) which was completely resolved after hospitalization. There were no changes in laboratory tests to indicate any liver or kidney toxicity and pulmonary function did not change.
Dasatinib has been FDA approved since 2006[1], and is currently approved for treating chronic Philadelphia chromosome-positive (Ph+) myeloid leukemia in both adults[2] and children[3].
The drug label for SPRYCEL describes known side effects of use for dasatinib, although it is limited mainly to use in cancer patients: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021986s7s8lbl.pdf
.
Literature Cited
1. Drug Approval Package: Sprycel (Dasatinib) NDA #021986 & 022072. (n.d.). Retrieved April 4, 2022, from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021986_022072_SprycelTOC.cfm