Name: Alpha Ketoglutarate (AKG)
Class: Supplement
Alias(es): CAS# 64-15-3, 2-Oxoglutaric acid, Oxoglutarate, Brands:
Background: α-ketoglutarate (AKG) is a key intermediate molecule that is involved in several cellular metabolic processes. Perhaps the most significant of these is the Citric Acid Cycle, an important pathway by which stored energy from carbs, fats, and proteins are released for use by cells. AKG first gained attention as a potential anti-aging treatment in 2014, when a study, published in the journal Nature, demonstrated that its administration in adult C. elegans (a type of microscopic worm) resulted in a 50% increase in lifespan. There are many potential mechanisms by which AKG exerts its effects, and it is known that AKG levels naturally increase in response to dietary restriction (DR). The 2014 study showed that increased α-KG levels inhibit ATP production, leading to less ATP, a decrease in oxygen consumption, and increased autophagy in both C. elegans and mammalian cells. Therefore, AKG may mimic DR and extend lifespan in a similar fashion to DR.
Is there evidence that AKG treats aging in human clinical studies?
ClinicalTrials.gov ID And Reference | Trial Status | Title | Summary | Result |
Completed | N/A | A retrospective analysis of DNA methylation (TruAge) age in 42 individuals taking Rejuvant®, an alpha-ketoglutarate based formulation, for an average period of 7 months. | -Preliminary assessment of the independent effect of CaAKG: By end of treatment, select subset of subjects were 7.69 years younger than at baseline. (P=7.263×10-5) –Entire cohort tested a mean of 7.96 years younger than at baseline. (P=6.538×10-12) Conclusion: the effect of supplementation with Rejuvant® decreases epigenetic age in a statistically significant manner in males and females | |
Active, Not Recruiting | Rejuvant™ Safety and Biomarker Study | Randomized, double-blind, placebo-controlled trial in adult men ages 45-65 years and postmenopausal women up to age 65 years to assess the anti-inflammatory (via serum CRP levels) properties, safety (via blood chemistry results and reports of adverse effects), and biological ages of participants (via saliva samples). | Not yet available. |
Is there evidence it works in preclinical studies for aging?
Model | Vivo/Vitro | Outcome | Reference |
C. elegans | In Vivo | 50% increase in lifespan and delayed decline in functional body movement w/o reducing food intake or body weight | |
Drosophila m. | In Vivo | Dose dependent increase in maximum lifespan extended in males, median and maximum lifespan extended in females. Side-effect: reduced reproductive performance. No effect on food intake or body weight. | |
Aged mice | In Vivo |
| |
Mice | In Vitro | αKG supplementation maintains the pluripotency of embryonic stem cells (ESCs) | |
Aged and aging Mice | In Vivo, In vitro |
| |
Mice (aged) | In vivo, in vitro |
|
AKG Mechanisms:
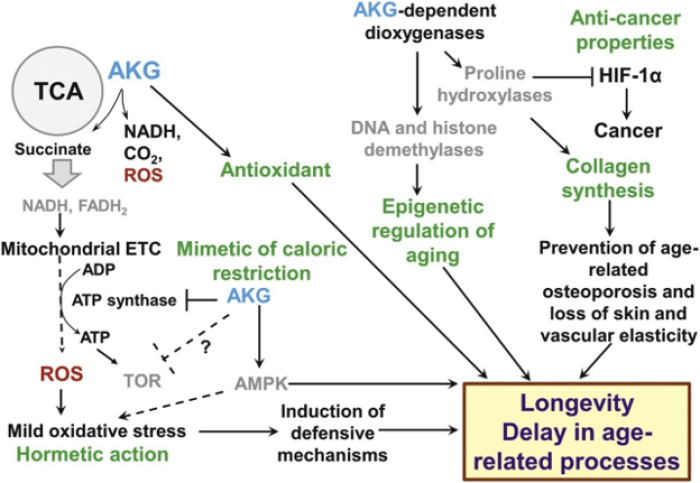
The diagram above is from a 2021 review article by Bayliak et al., “Pleiotropic effects of alpha-ketoglutarate as a potential anti-ageing agent,” published in the journal Ageing Research Reviews. It describes the many potential mechanisms by which AKG supplementation delays the effects of aging and increases lifespan, based on studies in model organisms. The green text summarizes each of the pathways by which AKG exerts its effects: hormetic action (where introduction of a small amount of a toxin, in this case ROS in the mitochondria, triggers a defensive beneficial response), mimicking dietary restriction, acting as an antioxidant, altering epigenetic elements, exerting anti-cancer effects by inhibiting HIF-1alpha, and triggering collagen synthesis.
Are there known safety concerns?
Limited human studies available, but currently no safety concerns have been reported in people. According to manufacturers, AKG and AKG salts have been grandfathered in under the umbrella of Generally Recognized As Safe (“GRAS”) compounds, because these compounds were sold as supplements prior to the Dietary Supplement Health and Education Act of 1994 (“DSHEA”).
Acute toxicity: A toxicity study of AKG administered to winstar rats found the LD50 was greater than 5 g/kg. Additionally, although there were some changes in physiological parameters at 4 g/kg, they were not sufficient to merit concern.
14-day toxicity: Another toxicity study tested 14 days of repeated oral administration at low (1.0 g/kg), middle (2.0 g/kg) and high (4.0 g/kg) doses of A-KG in Wistar rats, after which rats were monitored for 7 days.
The study determined that the No Observed Adverse Effect Level (NOAEL) for 14-days is 1 g/kg body weight for both sexes.
The high dose had clinical effects such as diarrhea (both sexes), decreased body weight (males), and anemia which resolved at end of treatment (females).
At both 2 g/kg and 4 g/kg, males experienced a delayed effect that suggested toxicity (statistically significant increases in weights of adrenal, liver, and kidney organs, and hematological changes).