Name: Rapamycin
Class: Drug (immunosuppressant)
Aliases: CAS# 53123-88-9, Sirolimus, Rapamune
Background: Rapamycin, also known by its trade names sirolimus and rapamune, has been FDA approved since 1999 as an anti-rejection drug for kidney transplant patients. However, since the 1980s, it has been known that rapamycin inhibits the mechanistic Target of Rapamycin (mTOR), a key regulator of cellular energy metabolism and growth[1]. More recently, in 2009, it was discovered that it was by this mechanism, attenuating the deregulated nutrient sensing hallmark of aging by inhibiting mTOR, that rapamycin extended the lifespan of mice, even when administered late in life[2]. Thereafter, a flurry of preclinical studies have been performed on rapamycin as a treatment for age-related diseases and for extending lifespan, many with promising results.
Is there evidence for Rapamycin in human clinical studies?
All the below trials accept healthy volunteers, though they have different minimum age requirements.
Completed Clinical Trials:
Pilot Study NCT02874924 (completed September 2018) on effects of rapamycin on aging in healthy elderly humans. 8-week study evaluating 1mg of rapamycin taken daily for 8 weeks vs. placebo.
Results: Limited results, as this is a safety trial. However, preliminary data revealed that T-cell assay did not show improvement in T-cell counts, and no improvement was seen in walking speed.
Currently Active, Not Recruiting Clinical Trials:
NCT04608448: Early phase 1 placebo controlled trial. Summary: Topical Rapamycin ointment will be applied to participant forearms to test whether epigenetic changes in the skin are elicited.
Currently Recruiting Trials:
NCT04742777: Phase 2 trial. Follow Up of pilot study NCT02874924 listed above. Summary: A translational trial to test whether rapamycin also improves life functions in humans focusing on elderly persons (aged 70-95).
NCT04488601: Phase 2 trial. Summary: This is a randomized, placebo-controlled trial into the safety and efficacy in reducing clinical measures of aging in an older adult population.
New Clinical Trials (Not Yet Recruiting):
NCT04994561: Pilot Study to Test the Safety and Efficacy of Metformin, Dasatinib, Rapamycin and Nutritional Supplements (Bio-quercetin; Bio-fisetin; Glucosamine; Nicotinamide Riboside; Trans-resveratrol) in Reducing Clinical Measures of Aging in Older Adults
NCT05237687: Phase 2 trial. A randomized control trial in the elderly to investigate whether sirolimus can reduce the occurrence or increase in biomarkers of aging processes.
Is there evidence it works in preclinical studies for aging?
https://www.aging-us.com/article/101976/text In human neonatal keratinocytes (a type of skin cell), rapamycin administered in vitro was found to effectively slow epigenetic aging.
https://link.springer.com/article/10.1007/s11357-021-00438-7 Rapamycin administered in vivo did not show any effect on epigenetic aging of marmoset blood.
Lifespan Extension from model organism studies:
Increased C. Elegans (a microscopic worm) lifespan by 19%. See figure 6a in https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3348514/
Increased D. Melanogaster (fruit fly) lifespan by 14.5%. See figure 1c in https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5348310/
Increased M. musculus (mouse) lifespan by 23% in males and 26% in females. See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4032600/
See table 1 below from a 2021 review paper by Zhang et al. for a list of organ-specific anti-aging effects of rapamycin seen in preclinical studies.
See Effect of rapamycin on aging and age-related diseases—past and future. This 2020 review paper, published in the journal GeroScience, details many of the studies of rapamycin as a treatment for aging and age-related diseases in both humans and in model organisms. Highlight from paper: ~90% of studies showed significantly increased lifespan in mice. See the Table of studies from the same paper, that lists studies where increases in lifespan in healthy mice with rapamycin treatment were found. A minority of the studies found no change or a significant decrease in lifespan, usually when certain mice with certain diseases were treated with rapamycin. See linked paper for details on that.
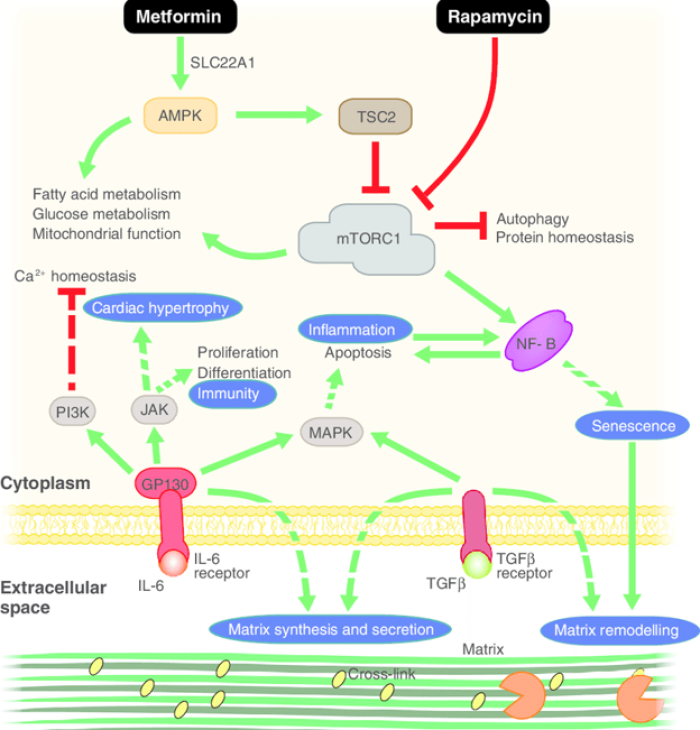
Mechanism: Below is “Figure 2” from a 2016 paper by Mallikarjun et al. from the journal EBioMedicine on repurposing old drugs for aging:
Caption: Mechanisms of promising repurposed anti-ageing drugs. Metformin and rapamycin are thought to act synergistically to improve protein and energy homeostasis. Inhibitors of IL-6 and NF-κB could act to reverse systemic inflammation and TFGβ inhibitors have been shown to reverse aberrant signalling caused by an aged stem cell niche (of which extracellular matrix is a major component). Green arrows indicate stimulation of the indicated process. Red, flat arrows indicate inhibition. Indirect interactions are indicated by dashed arrows. Processes that have effects at the tissue or organ level are surrounded by blue circles.
Are there known safety concerns?
See List of side effects on Mayo Clinic website (when used for chronic immunosuppression at much higher dosages than are typically used for aging).
The FDA drug label for Rapamune describes known side effects of use for rapamycin, although it is limited mainly to use in kidney transplant patients.
Additional human side effects are listed in this review article by Zhang et al. 2021: See table below from article for a summary:
In mice, side effects reported in (~1 year) rapamycin feeding studies include testicular atrophy, accelerated cataract formation, and glucose insensitivity[3,4].
Non-human toxicity excerpts from Pubchem: Reproductive toxicity has been observed in male rats and monkeys, and dose-dependent increases in carcinogenicity were observed in female mice.
Article suggesting ways to potentially mitigate side effects: They suggest a schedule of intermittent dosing with rapamycin or using the FDA-approved rapamycin analogs everolimus and temsirolimus instead.
Literature Cited:
1. Selvarani, R., Mohammed, S., & Richardson, A. (2021). Effect of rapamycin on aging and age-related diseases—Past and future. GeroScience, 43(3), 1135–1158. https://doi.org/10.1007/s11357-020-00274-1
2. Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., Nadon, N. L., Wilkinson, J. E., Frenkel, K., Carter, C. S., Pahor, M., Javors, M. A., Fernandez, E., & Miller, R. A. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392–395. https://doi.org/10.1038/nature08221